C102814
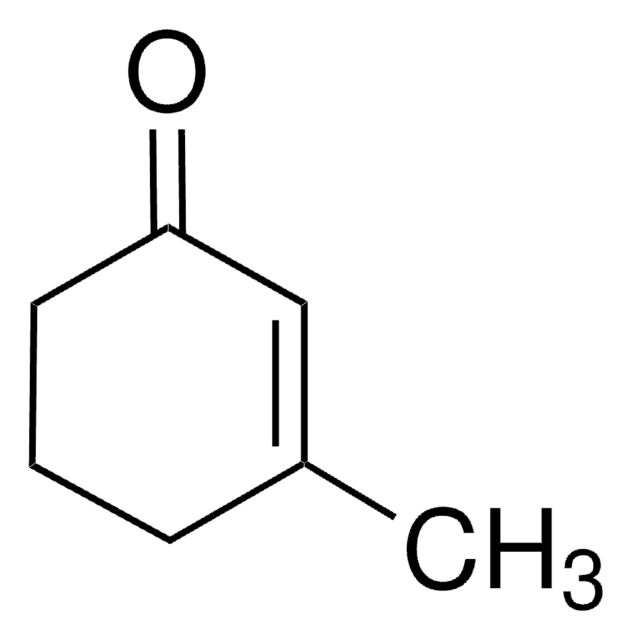
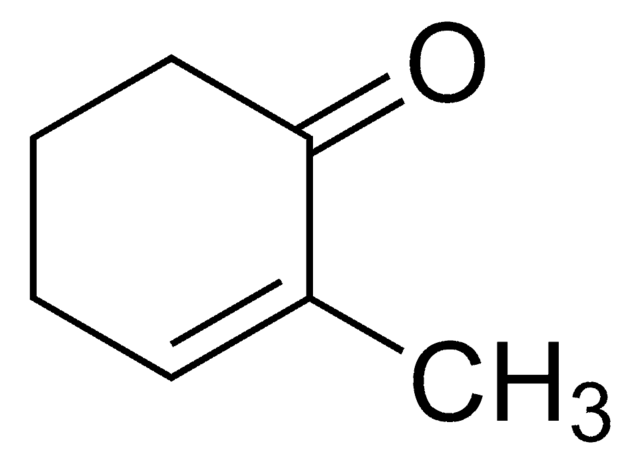
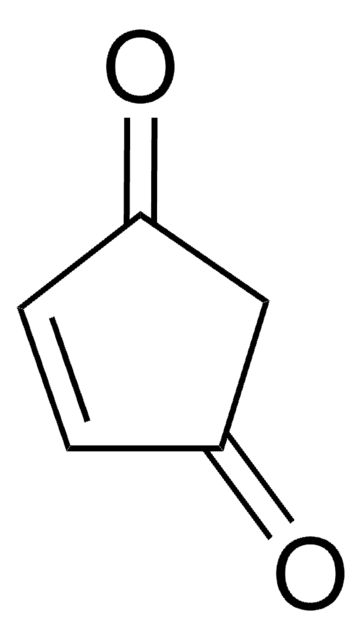
2-Cyclohexen-1-one
≥95%
동의어(들):
1-Cyclohexen-3-one, 2-Cyclohexenone, 3-Oxocyclohexene, Cyclohexen-3-one, Cyclohexenone
로그인조직 및 계약 가격 보기
크기 선택
모든 사진(1)
크기 선택
보기 변경
About This Item
Linear Formula:
C6H8(=O)
CAS Number:
Molecular Weight:
96.13
Beilstein:
1280477
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
vapor pressure
760 mmHg ( 168 °C)
분석
≥95%
양식
liquid
refractive index
n20/D 1.488 (lit.)
bp
171-173 °C (lit.)
mp
−53 °C (lit.)
density
0.993 g/mL at 25 °C (lit.)
SMILES string
O=C1CCCC=C1
InChI
1S/C6H8O/c7-6-4-2-1-3-5-6/h2,4H,1,3,5H2
InChI key
FWFSEYBSWVRWGL-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Versatile electrophile employed in a range of addition reactions including conjugate addition of organocopper nucleophiles, Michael reaction with enol silanes, and phosphoniosilylations.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 2 Dermal - Acute Tox. 3 Oral - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
132.8 °F - closed cup
Flash Point (°C)
56 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Tetrahedron Letters, 34, 3881-3881 (1993)
Tetrahedron Asymmetry, 4, 2427-2427 (1993)
Albert Poater et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(48), 14348-14353 (2010-11-18)
The current approach to improve and tune the enantioselective performances of transition-metal catalysts for asymmetric synthesis is mostly focused to modifications of the steric properties of the ancillary ligands of the active metal. Nevertheless, it is also known that electrostatic
Masaki Okamoto et al.
Chemical communications (Cambridge, England), (47)(47), 7363-7365 (2009-12-22)
Conjugate addition of Et(2)Zn to 2-cyclohexen-1-one catalyzed by Cu(OTf)(2) combined with an azolium salt derived from (S)-leucinol produced the corresponding (S)-adduct, while the use of Cu(acac)(2) in combination with the same ligand afforded the (R)-adduct as a major product.
M Teresa Barros et al.
Chemical communications (Cambridge, England), 48(88), 10901-10903 (2012-10-02)
An iodine mediated aromatization leading to a one-pot synthesis of iodo-N-arylanilines and N-arylanilines is reported. This highly regioselective aliphatic-aromatic transformation can be performed with various combinations of 2-cyclohexenones and anilines. The presence of a directing group is crucial for achieving
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.