C112909
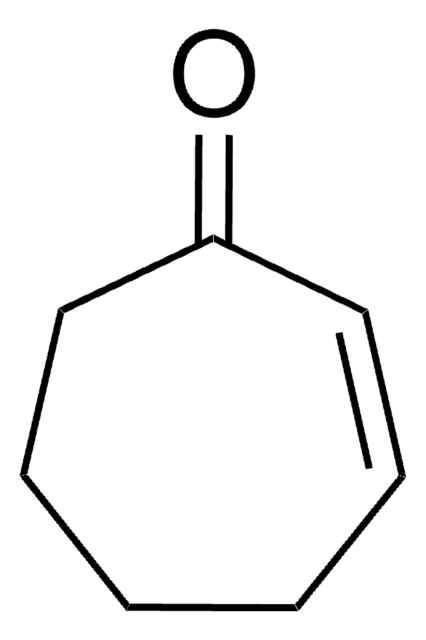
2-Cyclopenten-1-one
98%
Synonym(s):
1-Cyclopenten-3-one, 1-Cyclopenten-5-one, 2-Cyclopentenone, Cyclopent-2-en-1-one, Cyclopenten-3-one
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H6O
CAS Number:
Molecular Weight:
82.10
Beilstein/REAXYS Number:
1446054
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
98%
form
liquid
refractive index
n20/D 1.481 (lit.)
bp
64-65 °C/19 mmHg (lit.)
density
0.98 g/mL at 25 °C (lit.)
Storage temp.
2-8°C
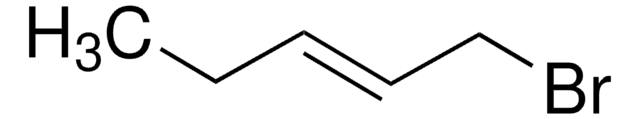
SMILES string
O=C1CCC=C1
InChI
1S/C5H6O/c6-5-3-1-2-4-5/h1,3H,2,4H2
Inchi Key
BZKFMUIJRXWWQK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Versatile electrophile employed in a variety of addition reactions including conjugate addition of organocopper nucleophiles, Michael reaction with silyl enol ethers, and siloxanes, Diels-Alder cycloadditions, and phosphoniosilylations.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron Letters, 34, 6777-6777 (1993)
Marc Revés et al.
Organic letters, 14(13), 3534-3537 (2012-06-28)
1,2,3,4-Tetramethyl-bicyclo[2.2.1]hepta-2,5-diene (TMNBD, for tetramethylnorbornadiene) has been prepared and used successfully as an acetylene equivalent in the synthesis of substituted cyclopentenones. TMNBD is easily accessible on a multigram scale and displays excellent reactivity toward the intermolecular Pauson-Khand reaction. Conjugate additions on
Filippo De Simone et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(51), 14527-14538 (2011-11-25)
The Nazarov cyclization of divinyl ketones gives access to cyclopentenones. Replacing one of the vinyl groups by a cyclopropane leads to a formal homo-Nazarov process for the synthesis of cyclohexenones. In contrast to the Nazarov reaction, the cyclization of vinyl-cyclopropyl
Brett D Schwartz et al.
Organic letters, 15(8), 1934-1937 (2013-04-05)
The title natural product, 1, has been synthesized in 20 steps from the enantiomerically pure cis-1,2-dihydrocatechol 2, itself obtained through the whole-cell biotransformation of toluene. The pivotal steps in the reaction sequence involve a Diels-Alder cycloaddition reaction between diene 2
Diverse reactivity in a rhodium(III)-catalyzed oxidative coupling of N-allyl arenesulfonamides with alkynes.
Dongqi Wang et al.
Angewandte Chemie (International ed. in English), 51(49), 12348-12352 (2012-10-31)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service