추천 제품
분석
98%
양식
crystals
bp
217-219 °C (lit.)
mp
43-46 °C (lit.)
저장 온도
2-8°C
SMILES string
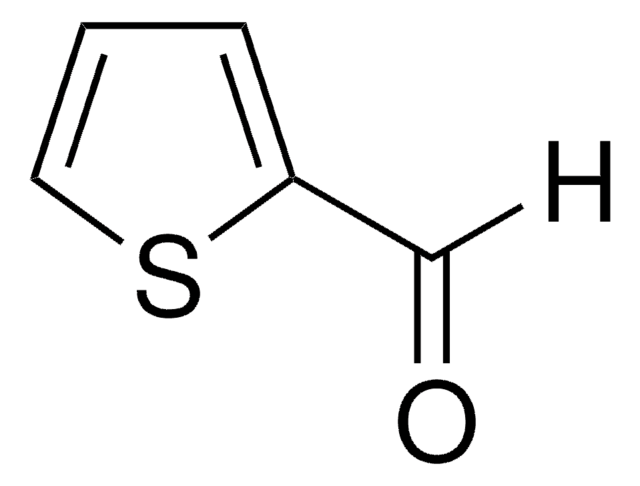
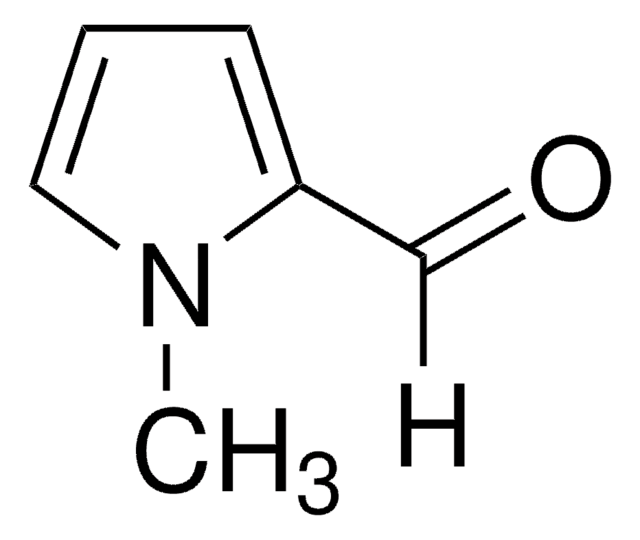
[H]C(=O)c1ccc[nH]1
InChI
1S/C5H5NO/c7-4-5-2-1-3-6-5/h1-4,6H
InChI key
ZSKGQVFRTSEPJT-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Pyrrole-2-carboxaldehydesis a heterocyclic building blocks characterized by a pyrrole ring with a formylgroup attached at the 2-position used in the production of various biologicallyactive compounds. Highly functionalized pyrrole-2-carboxaldehydes have beenutilized as an intermediate in the creation of oligopyrrole macrocycles.
애플리케이션
- Pyrimidine-based functional fluorescent organic nanoparticle probe for detection of Pseudomonas aeruginosa.: This study used pyrrole-2-carboxaldehyde to develop a fluorescent nanoparticle probe based on pyrimidine for detecting Pseudomonas aeruginosa, enhancing diagnostic capabilities in microbiology (Kaur G et al., 2015).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
224.6 °F - closed cup
Flash Point (°C)
107 °C - closed cup
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Corey A Rice et al.
The Journal of chemical physics, 126(13), 134313-134313 (2007-04-14)
Intermolecular interactions relevant for antiparallel beta-sheet formation between peptide strands are studied by Fourier transform infrared spectroscopy of the low temperature, vacuum-isolated model compound pyrrole-2-carboxaldehyde and its dimer in the N-H and C=O stretching range. Comparison to quantum chemical predictions
Takumi Ishizuka et al.
Chemical communications (Cambridge, England), 48(88), 10835-10837 (2012-10-04)
Toward new biotechnology by genetic alphabet expansion, we developed an efficient site-specific labeling method for large RNA molecules. The combination of unnatural base pair transcription and post-transcriptional modification by click chemistry enables simple RNA labeling with a wide variety of
Michiko Kimoto et al.
Nucleic acids research, 35(16), 5360-5369 (2007-08-19)
Fluorescent labeling of nucleic acids is widely used in basic research and medical applications. We describe the efficient site-specific incorporation of a fluorescent base analog, 2-amino-6-(2-thienyl)purine (s), into RNA by transcription mediated by an unnatural base pair between s and
Tsuneo Mitsui et al.
Journal of the American Chemical Society, 125(18), 5298-5307 (2003-05-02)
An unnatural hydrophobic base, pyrrole-2-carbaldehyde (denoted as Pa), was developed as a specific pairing partner of 9-methylimidazo[(4,5)-b]pyridine (Q). The Q base is known to pair with 2,4-difluorotoluene (F) as an isostere of the A-T pair, and F also pairs with
Yasushi Hikida et al.
Nature protocols, 5(7), 1312-1323 (2010-07-03)
Methods for fluorescent probing at a defined position of RNA provide powerful tools for analyzing the local structural conformation of functional RNA molecules by tracking fluorescence changes. In this article, we describe the site-specific fluorescent probing of RNA by transcription
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.