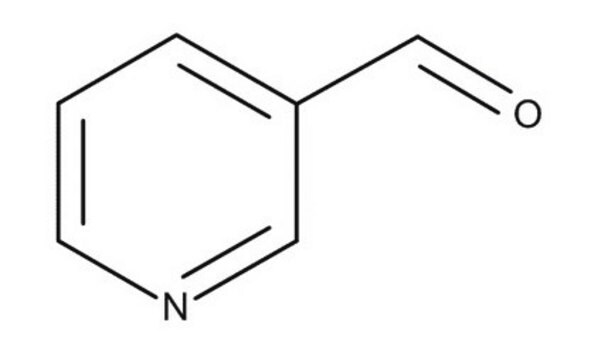
P62402
4-Pyridinecarboxaldehyde
97%
Synonym(s):
Isonicotinaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C6H5NO
CAS Number:
Molecular Weight:
107.11
Beilstein/REAXYS Number:
105342
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
assay
97%
refractive index
n20/D 1.544 (lit.)
bp
71-73 °C/10 mmHg (lit.)
density
1.137 g/mL at 20 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]C(=O)c1ccncc1
InChI
1S/C6H5NO/c8-5-6-1-3-7-4-2-6/h1-5H
InChI key
BGUWFUQJCDRPTL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Pyridinecarboxaldehyde is a heterocyclic building block used to prepare Schiff bases via a Korich-type reaction.
Application
4-Pyridinecarboxaldehyde can be used for the synthesis of:
- ʅ,β-Unsaturated amides by coupling with N,N-disubstituted formamides.
- meso-Substituted A3-corroles.
- N-(4-pyridylmethyl)-L-valine as a ligand to construct zinc metal–organic frameworks (Zn-MOFs).
- 4′-Pyridyl terpyridines, with potential application as anticancer and antimicrobial agents.
- 4-pyridinecarboxaldehyde thiosemicarbazone, as a corrosion inhibitor for mild steel.
signalword
Danger
hcodes
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 1
flash_point_f
172.0 °F
flash_point_c
77.8 °C
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A combined computational/experimental study on HSA binding of two water-soluble Schiff base ligands derived from pyridine derivative and ethylendiamine.
Hajar Molaee et al.
Journal of biomolecular structure & dynamics, 37(3), 641-648 (2018-02-03)
Cross coupling of acyl and aminyl radicals: Direct synthesis of amides catalyzed by Bu4NI with TBHP as an oxidant.
Liu Z, et al.
Angewandte Chemie (International Edition in English), 51(13), 3231-3235 (2012)
Helical water chain mediated proton conductivity in homochiral metal-organic frameworks with unprecedented zeolitic unh-topology.
Sahoo SC, et al.
Journal of the American Chemical Society, 133(44), 17950-17958 (2011)
Sakineh Omidi et al.
Carbohydrate polymers, 208, 477-485 (2019-01-20)
Chitosan is an antibacterial biopolymer and conjugation of it with other antimicrobial agents can be a valuable method to improve the potential application of the resultant materials in the various industries such as cosmetics, food and packaging materials. In this
Copper (I) iodide complex with 4-pyridinecarboxaldehyde ligand: Synthesis, spectroscopic characterisation, AIM and NCI analysis combined with molecular docking and antibacterial activity studies
Celik S, et al.
Journal of Molecular Structure, 134279, 1273-1273 (2023)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![Benzo[b]thiophene-2-carboxaldehyde 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)