T90301
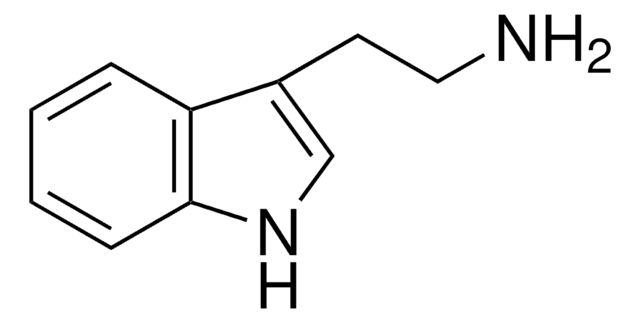
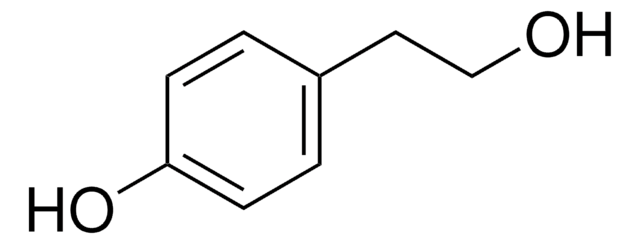
3-(2-Hydroxyethyl)indole
97%
동의어(들):
2-(3-Indolyl)ethanol, 3-Indoleethanol, IEA, NSC 3884, Tryptophol
크기 선택
About This Item
추천 제품
Quality Level
분석
97%
mp
56-59 °C (lit.)
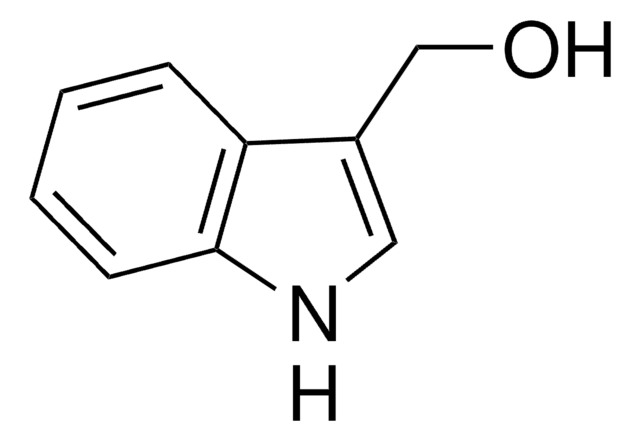
SMILES string
OCCc1c[nH]c2ccccc12
InChI
1S/C10H11NO/c12-6-5-8-7-11-10-4-2-1-3-9(8)10/h1-4,7,11-12H,5-6H2
InChI key
MBBOMCVGYCRMEA-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
1 of 4
이 품목 | 152757 | 565032 | H48808 |
|---|---|---|---|
| Quality Level 100 | Quality Level 200 | Quality Level 100 | Quality Level 100 |
| mp 161-163 °C (lit.) | mp 119-122 °C (lit.) | mp 158-162 °C (lit.) | mp 188-191 °C (lit.) |
애플리케이션
- Inhibitors of the C-terminal domain of RNA polymerase II and their antitumor activities
- Anti-HIV-1 agents
- Inhibitors of Protein-Protein Interactions
- Partial agonists of the serotonin 5-HT1A receptor
- Growth hormone secretagogues
- Vascular endothelial growth factor (VEGF) inhibitors
- A2B adenosine receptor ligands
- Potential detoxification inhibitors of the crucifer phytoalexin brassinin
- Inhibitors of interleukine 6
- Dual binding site acetylcholinesterase inhibitors
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.