추천 제품
설명
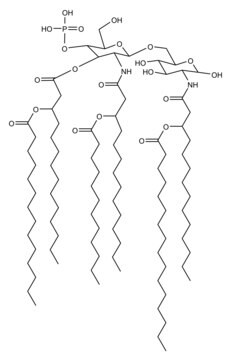
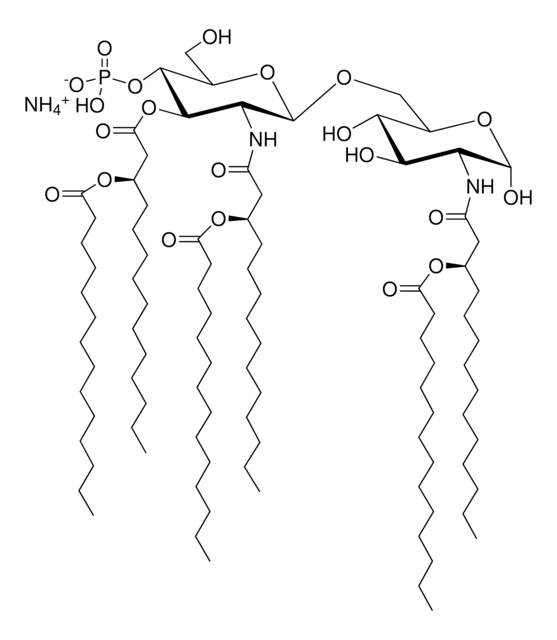
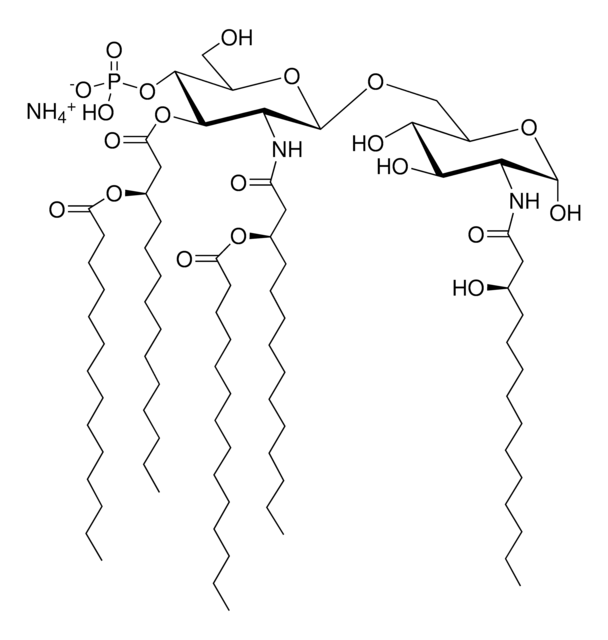
Monophosphoryl Lipid A (Synthetic) (PHAD™)
분석
>99% (HPLC)
양식
powder
포장
pkg of 1 × 1 mg (699800P-1mg)
pkg of 1 × 5 mg (699800P-5mg)
제조업체/상표
Avanti Research™ - A Croda Brand
응용 분야
vaccine development
배송 상태
dry ice
저장 온도
−20°C
일반 설명
Monophosphoryl lipid A (MPLA) is either extracted from bacterial lipid A or by chemical synthesis.
Vaccination is well-accepted as an effective method to prevent infections by mounting pathogen-specific immune responses prior to the infection. Usually, immunization with vaccine antigens alone is not able to induce robust or long-lasting immune responses — resulting in failure of protective immunity against infections. Thus, adjuvants are required to enhance cellular or humoral immune responses upon immunization. Because vaccine adjuvants using Lipid A have proven to be safe and effective in inducing Th-1 type immune responses to heterologous proteins in animal and human vaccines, Avanti developed Phosphorylated HexaAcyl Disaccharide (PHAD™), the first fully synthetic monophosphoryl Lipid A available for use as an adjuvant in human vaccines.
애플리케이션
MPLA PHAD™ has been used:
- as a component of cobalt porphyrin-phospholipid (Co-PoP) liposomes for the immunization of mice with membrane proximal external region (MPER) of the gp41 envelope protein
- as an adjuvant along with dimethyldioctadecylammonium bromide(DDA) for C. muridarum recombinant membrane protein based multi-subunit vaccine
- as toll-like receptor-4 (TLR4) agonist adjuvant for respiratory syncytial virus (RSV) fusion (F) protein FI-RSV vaccine
생화학적/생리학적 작용
Monophosphoryl lipid A (MPLA) is a natural agonist for the toll-like receptor-4 (TLR4). It is useful as an adjuvant in immunization. MPLA is a safe prophylactic agent and has immunotherapeutic applications. MPLA used in vaccination improves B cell and T cell-mediated immunity.
포장
2 mL Amber Glass Crimp Cap Vial (699800P-1mg)
2 mL Amber Glass Crimp Cap Vial (699800P-5mg)
기타 정보
For R&D use only. Not for drug, household, or other uses.
법적 정보
Avanti Research is a trademark of Avanti Polar Lipids, LLC
PHAD is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Caroline Boudousquié et al.
Vaccines, 8(1) (2020-01-18)
With the emergence of immune checkpoint inhibitors and adoptive T-cell therapies, there is a considerable interest in using personalized autologous dendritic cell (DC) vaccines in combination with T cell-targeting immunotherapies to potentially maximize the therapeutic impact of DC vaccines. Here
Susana Lousada-Dietrich et al.
Vaccine, 29(17), 3284-3292 (2011-02-26)
GMZ2 adjuvanted by aluminum hydroxide is a candidate malaria vaccine that has successfully passed phase 1 clinical testing in adult German and Gabonese volunteers and Gabonese children under five. Here we report a preclinical study screening a series of adjuvant
Katharina Richard et al.
Vaccine, 38(27), 4298-4308 (2020-05-12)
Toll-like receptors (TLRs), a family of "pattern recognition receptors," bind microbial and host-derived molecules, leading to intracellular signaling and proinflammatory gene expression. TLR4 is unique in that ligand-mediated activation requires the co-receptor myeloid differentiation 2 (MD2) to initiate two signaling
Functionalization of cobalt porphyrin-phospholipid bilayers with his-tagged ligands and antigens
Shao S, et al.
Nature Chemistry, 7(5), 438-438 (2015)
Rhea N Coler et al.
PloS one, 6(1), e16333-e16333 (2011-02-08)
Innate immune responses to vaccine adjuvants based on lipopolysaccharide (LPS), a component of gram-negative bacterial cell walls, are driven by Toll-like receptor (TLR) 4 and adaptor proteins including MyD88 and TRIF, leading to the production of inflammatory cytokines, type I
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.