크기 선택
모든 사진(1)
크기 선택
보기 변경
About This Item
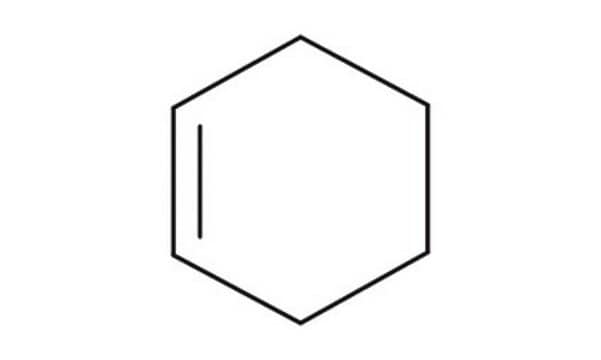
실험식(Hill 표기법):
C6H10
CAS Number:
Molecular Weight:
82.14
Beilstein:
906737
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.21
bp:
83 °C (lit.)
vapor pressure:
160 mmHg ( 20 °C)
추천 제품
vapor density
2.8 (vs air)
Quality Level
vapor pressure
160 mmHg ( 20 °C)
분석
≥99.0%
autoignition temp.
590 °F
포함
100 ppm BHT as inhibitor
expl. lim.
5 %
증발 잔류물
≤0.01%
refractive index
n20/D 1.446 (lit.)
n20/D 1.446
bp
83 °C (lit.)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Cyclohexene has been used in the green synthesis of cyclohexene oxide via hydrogen peroxide epoxidation in glycerol-based solvents using bis[3,5-bis(trifluoromethyl)-diphenyl] diselenide as a precatalyst. It can also undergo hydrogen peroxide oxidation in the presence of peroxytungstate-oxalic acid complex catalyst to form adipic acid.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Asp. Tox. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
1.4 °F
Flash Point (°C)
-17 °C
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Epoxidation of cyclooctene and cyclohexene with hydrogen peroxide catalyzed by bis [3, 5-bis (trifluoromethyl)-diphenyl] diselenide: Recyclable catalyst-containing phases through the use of glycerol-derived solvents
Garcia-Marin H, et al.
J. Mol. Catal. A: Chem., 334(1), 83-88 (2011)
Catalytic hydrogenation of cyclohexene: 3. Gas-phase and liquid-phase reaction on supported palladium.
Gonzo EE and Boudart M.
J. Catal., 52(3), 462-471 (1978)
Hydration of cyclohexene with solid acid catalysts.
Zhang H, et al.
Chemical Engineering Science, 57(2), 315-322 (2002)
Partial liquid phase hydrogenation of benzene to cyclohexene over ruthenium catalysts in the presence of an aqueous salt solution: I. Preparation, characterization of the catalyst and study of a number of process variables.
Struijk J, et al.
Applied Catalysis A: General, 83(2), 263-295 (1992)
Hikaru Yanai et al.
Chemical communications (Cambridge, England), 47(25), 7245-7247 (2011-05-25)
In the absence of any additional catalysts, the reactions of (CF(3)SO)(2)CH(2), aldehydes, and 1,3-dienes gave gem-bis(triflyl)cyclohexenes in excellent yields with high regioselectivity. gem-Bis(triflyl)cyclohexene products can be easily converted to the corresponding aryl trifluoromethyl sulfones.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.