크기 선택
모든 사진(1)
크기 선택
보기 변경
About This Item
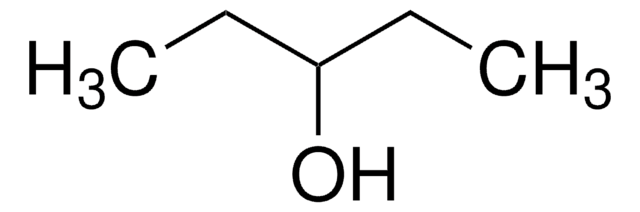
Linear Formula:
CH3(CH2)4OH
CAS Number:
Molecular Weight:
88.15
Beilstein:
1730975
EC Number:
MDL number:
UNSPSC 코드:
12352001
PubChem Substance ID:
NACRES:
NA.21
bp:
136-138 °C (lit.)
vapor pressure:
1 mmHg ( 13.6 °C)
1.5 mmHg ( 20 °C)
1.5 mmHg ( 20 °C)
추천 제품
vapor density
3 (vs air)
Quality Level
vapor pressure
1 mmHg ( 13.6 °C)
1.5 mmHg ( 20 °C)
제품 라인
ReagentPlus®
분석
≥99%
양식
liquid
autoignition temp.
572 °F
expl. lim.
10 %, 100 °F
dilution
(for general lab use)
refractive index
n20/D 1.409 (lit.)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
1-Pentanol is a linear alcohol. Pentane, a ″next generation″ alcohol fuel, is a promising substitute for conventional gasoline and diesel fuels. Kinetics of thermal decomposition of its isomeric forms (1-pentanol, 2-methyl-1-butanol, and 3-methyl-1-butanol) have been reported.
1-Pentanol is a colorless liquid having a pleasant odor, which can be obtained as a product from the process of fractional distillation of mixed alcohols resulting from the chlorination and alkaline hydrolysis of pentane occurs.
1-Pentanol is a colorless liquid having a pleasant odor, which can be obtained as a product from the process of fractional distillation of mixed alcohols resulting from the chlorination and alkaline hydrolysis of pentane occurs.
애플리케이션
1-Pentanol may be used as a cosurfactant in the water-in-oil mixed surfactant microemulsions.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
120.2 °F - closed cup
Flash Point (°C)
49 °C - closed cup
이미 열람한 고객
Harbison.DR, et al.
Hamilton and Hardy's Industrial Toxicology, (6) (2015)
Long Zhao et al.
The journal of physical chemistry. A, 116(37), 9238-9244 (2012-08-23)
Pentanol is one of the promising "next generation" alcohol fuels with high energy density and low hygroscopicity. In the present work, dominant reaction channels of thermal decomposition of three isomers of pentanol: 1-pentanol, 2-methyl-1-butanol, and 3-methyl-1-butanol were investigated by CBS-QB3
Experimental and detailed kinetic modeling study of 1-pentanol oxidation in a JSR and combustion in a bomb.
Togbe C, et al.
Proceedings of the Combustion Institute, 33(1), 367-374 (2011)
Acid/base- and anion-controllable organogels formed from a urea-based molecular switch.
Sheng-Yao Hsueh et al.
Angewandte Chemie (International ed. in English), 49(48), 9170-9173 (2010-10-19)
Nadav Amdursky et al.
The journal of physical chemistry. A, 115(24), 6481-6487 (2011-05-19)
Time-resolved emission techniques were employed to study the nonradiative process of thioflavin-T (ThT) in 1-propanol, 1-butanol, and 1-pentanol as a function of the hydrostatic pressure. Elevated hydrostatic pressure increases the alcohol viscosity, which in turn strongly influences the nonradiative rate
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.