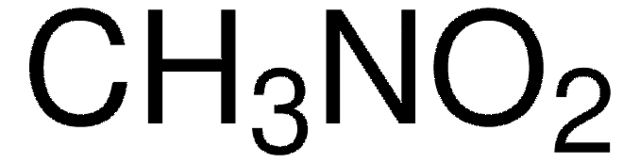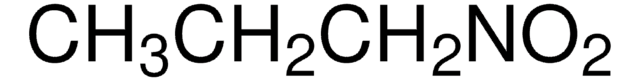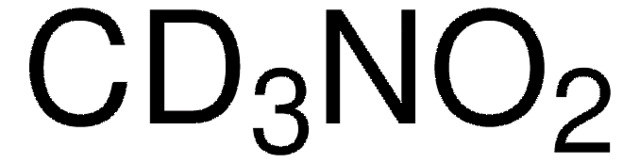모든 사진(1)
About This Item
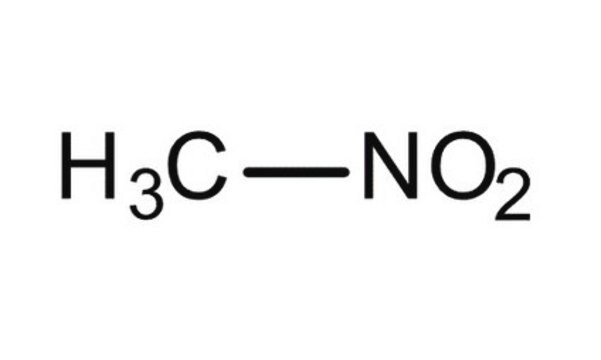
실험식(Hill 표기법):
CH3NO2
CAS Number:
Molecular Weight:
61.04
Beilstein:
1698205
EC Number:
MDL number:
UNSPSC 코드:
12352102
PubChem Substance ID:
NACRES:
NA.21
bp:
101.2 °C (lit.)
vapor pressure:
2.7 mmHg
가격 및 재고 정보를 현재 이용할 수 없음
추천 제품
vapor density
2.1 (vs air)
Quality Level
vapor pressure
2.7 mmHg
제품 라인
ReagentPlus®
분석
≥99.0%
양식
liquid
autoignition temp.
784 °F
expl. lim.
7.3 %, 33 °F
dilution
(for general lab use)
refractive index
n20/D 1.382 (lit.)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Nitromethane (CH3NO2) is the simplest nitro organic compound used for a wide range of applications, including as polar solvents to racing fuel. It serves as a solvent for organic chemistry and as a valuable building block for various useful compounds like nitroalkanes, β-nitroalcohols, nitroalkenes, carbonyl compounds, amines, and heterocycles. In industry, nitromethane can be used to stabilize halogenated hydrocarbons.
애플리케이션
Nitromethane can be used:
- As a reagent in the synthesis of 3-nitroindoles by arylation with N-(2-bromoaryl) imidates using palladium catalyst.
- As a cyanating reagent for the synthesis of thiocyanates in presence of base (KOAc) and iodine.
- In the preparation of cobalt cage complexes from polyamines and formaldehyde.
- As a solvent in the preparation of β-substituted γ-nitroaldehydes by enantioselective cross-coupling of β-arylated or γ,δ-unsaturated aldehydes using oxidizing agent DDQ (2,3-dichloro-5,6-dicyanoquinone) and catalyst diphenylprolinol silyl ether.
특징 및 장점
Nitromethane as high energy monopropellant exhibits
- Low toxicity.
- High specific impulse.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Flam. Liq. 3 - Repr. 2
Storage Class Code
4.1A - Other explosive hazardous materials
WGK
WGK 2
Flash Point (°F)
95.0 °F - closed cup
Flash Point (°C)
35 °C - closed cup
이미 열람한 고객
Metal ion encapsulation: cobalt cages derived from polyamines, formaldehyde, and nitromethane.
Geue RJ, et al.
Journal of the American Chemical Society, 106(19), 5478-5488 (1984)
The catalytic chemistry of nitromethane over Co-ZSM5 and other catalysts in connection with the methane-NOxSCR reaction.
Cowan AD, et al.
J. Catal., 176(2), 329-343 (1998)
Benedek Vakulya et al.
Organic letters, 7(10), 1967-1969 (2005-05-07)
Cinchona alkaloid-derived chiral bifunctional thiourea organocatalysts were synthesized and applied in the Michael addition between nitromethane and chalcones with high ee and chemical yields.
Huachang Hong et al.
The Science of the total environment, 444, 196-204 (2012-12-29)
The formations of THMs, HAAs, and HNMs from chlorination and chloramination of water from Jinlan Reservoir were investigated in this study. Results showed that monochloramine rather than chlorine generally resulted in lower concentration of DBPs, and the DBPs formation varied
Ryan R Walvoord et al.
Organic letters, 14(16), 4086-4089 (2012-07-31)
An efficient cross-coupling reaction of aryl halides and nitromethane was developed with the use of parallel microscale experimentation. The arylnitromethane products are precursors for numerous useful synthetic products. An efficient method for their direct conversion to the corresponding oximes and
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.