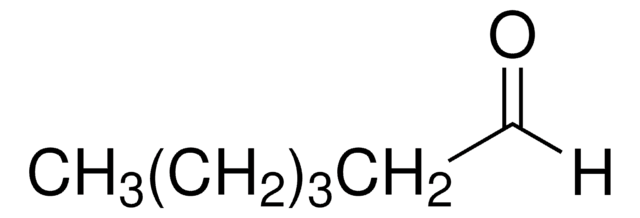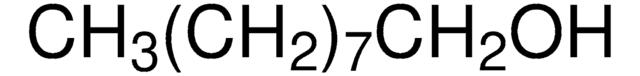모든 사진(2)
About This Item
Linear Formula:
CH3(CH2)5OH
CAS Number:
Molecular Weight:
102.17
Beilstein:
969167
EC Number:
MDL number:
UNSPSC 코드:
12352104
PubChem Substance ID:
NACRES:
NA.21
bp:
156-157 °C (lit.)
vapor pressure:
1 mmHg ( 25.6 °C)
추천 제품
Grade
reagent grade
Quality Level
vapor density
4.5 (vs air)
vapor pressure
1 mmHg ( 25.6 °C)
분석
98%
양식
liquid
autoignition temp.
559 °F
expl. lim.
0.34-6.3 %
dilution
(for analytical testing)
refractive index
n20/D 1.418 (lit.)
bp
156-157 °C (lit.)
mp
−52 °C (lit.)
density
0.814 g/mL at 25 °C (lit.)
SMILES string
CCCCCCO
InChI
1S/C6H14O/c1-2-3-4-5-6-7/h7H,2-6H2,1H3
InChI key
ZSIAUFGUXNUGDI-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
1-Hexanol is a linear primary alcohol, made up of six carbon atoms and is produced by the oligomerization of ethylene. There is a wide range of uses for 1-hexanol, including plasticizers, antiseptics, fragrances, pharmaceuticals, textiles, and leather finishing agents.
애플리케이션
1-Hexanol is used:
- As an oil phase in the preparation of manganese zinc ferrite nanoparticles by precipitation in reverse microemulsion system.
- As a solvent in the separation of carboxylic acids and tetrahydrofurfuryl alcohol from water.
- As a solvent in the synthesis of ZnO (zinc oxide) quantum particles from zinc acetate by precipitation method.
특징 및 장점
1-Hexanol or Hexyl alcohol exhibits high reactivity at a lower temperature due to the alkane-like reaction pathways that begin at a secondary carbon site.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
140.0 °F - closed cup
Flash Point (°C)
60 °C - closed cup
이미 열람한 고객
Erik Jue et al.
Scientific reports, 10(1), 1940-1940 (2020-02-08)
The success of fundamental and applied nucleic acid (NA) research depends on NA purity, but obtaining pure NAs from raw, unprocessed samples is challenging. Purification using solid-phase NA extractions utilizes sequential additions of lysis and wash buffers followed by elution.
Annie Handler et al.
Cell, 178(1), 60-75 (2019-06-25)
Animals rely on the relative timing of events in their environment to form and update predictive associations, but the molecular and circuit mechanisms for this temporal sensitivity remain incompletely understood. Here, we show that olfactory associations in Drosophila can be
Maria Eugenia Villar et al.
Cell reports, 30(8), 2603-2613 (2020-02-27)
Research on honeybee memory has led to a widely accepted model in which a single pairing of an odor stimulus with sucrose induces memories that are independent of protein synthesis but is unable to form protein-synthesis-dependent long-term memory (LTM). The
Justin T Mohr et al.
Journal of medicinal chemistry, 48(12), 4172-4176 (2005-06-10)
The polyhydroxyalkanes 1,6,11,16-hexadecanetetraol (1) and 2,7,12,17-octadecanetetraol (2) were synthesized utilizing the thiophene ring as a scaffold to affix the hydroxyalkyl chains by lithiation of the acidic alpha-hydrogens and subsequent desulfurization. Both compounds exhibited significant anesthetic potency, individually and in additivity
Mir Ali Farajzadeh et al.
Analytica chimica acta, 713, 70-78 (2011-12-28)
In the present work a new, simple, rapid and environmentally friendly dispersive liquid-liquid microextraction (DLLME) method has been developed for extraction/preconcentration of some triazole pesticides in aqueous samples and in grape juice. The extract was analyzed with gas chromatography-flame ionization
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.