A0262
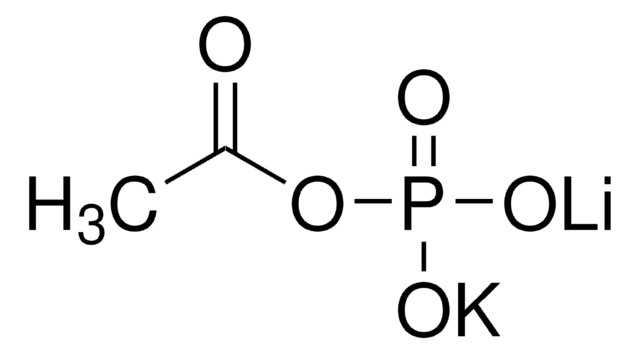
Lithium potassium acetyl phosphate
≥85% (elemental analysis), powder
동의어(들):
Acetyl phosphate lithium potassium salt
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
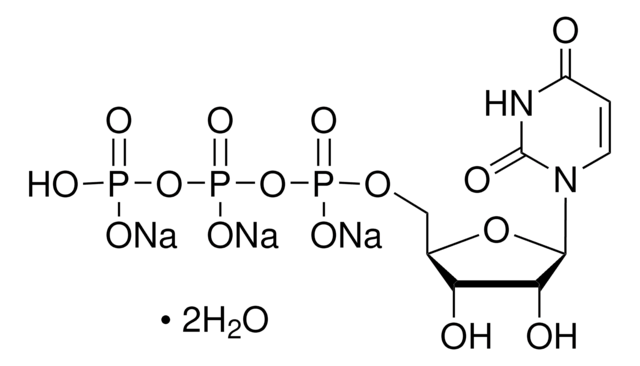
CH3COOP(O)(OK)(OLi)
CAS Number:
Molecular Weight:
184.06
Beilstein:
5689718
EC Number:
MDL number:
UNSPSC 코드:
12352204
PubChem Substance ID:
NACRES:
NA.32
추천 제품
제품명
Lithium potassium acetyl phosphate, high-energy phosphate donor
Quality Level
분석
≥85% (elemental analysis)
양식
powder
mp
>300 °C (lit.)
solubility
water: 25 mg/mL, clear, colorless
저장 온도
−20°C
SMILES string
[Li+].[K+].CC(=O)OP([O-])([O-])=O
InChI
1S/C2H5O5P.K.Li/c1-2(3)7-8(4,5)6;;/h1H3,(H2,4,5,6);;/q;2*+1/p-2
InChI key
RLQMPLKXFIXRCV-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
애플리케이션
Lithium potassium acetyl phosphate:
- has been used as a substrate for CpxR (transcription factor) in x-ray crystallography to soak CpxR receiver domain (CpxRRD) crystals to obtain the phosphorylated form
- may be used as a phosphate (P) donor in a study to screen a wide range of natural and synthetic organic phosphate donors with several enzymes on various hydroxyl?compounds for the improved enzymatic synthesis of phosphate monoesters
- has been used to set up premixA for in vitro translation reaction
생화학적/생리학적 작용
Lithium potassium acetyl phosphate is commonly used as the source for acetyl phosphate. So acetyl phosphate can be used as a suitable alternative to replace inorganic oligophosphates in phosphatase-catalyzed transphosphorylation to reduce inorganic monophosphate by-product formation.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Repr. 2 - Skin Corr. 1B
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Soma Mukhopadhyay et al.
Bioorganic chemistry, 36(2), 65-69 (2008-02-26)
Acetate kinase, a member of the ASKHA (Acetate and Sugar Kinases, Hsp70, Actin) phosphotransferase superfamily is a central enzyme in prokaryotic carbon and energy metabolism. Recently extensive structural and biochemical studies of acetate kinase and related carboxylate kinases have been
Gábor Tasnádi et al.
Advanced synthesis & catalysis, 360(12), 2394-2401 (2018-10-20)
Undesired product hydrolysis along with large amounts of waste in form of inorganic monophosphate by-product are the main obstacles associated with the use of pyrophosphate in the phosphatase-catalyzed synthesis of phosphate monoesters on large scale. In order to overcome both
Ariel E Mechaly et al.
Structure (London, England : 1993), 25(6), 939-944 (2017-05-30)
Bacterial two-component systems consist of a sensor histidine kinase (HK) and a response regulator (RR). HKs are homodimers that catalyze the autophosphorylation of a histidine residue and the subsequent phosphoryl transfer to its RR partner, triggering an adaptive response. How
Juan Andres Imelio et al.
Bio-protocol, 7(16), e2510-e2510 (2017-08-20)
We have developed protocols to generate site-specific variants of the histidine-kinase DesK and its cognate response regulator DesR, conducive to trapping different signaling states of the proteins. Co-expression of both partners in E. coli, ensuring an excess of the regulator
Sebastian R Schmidl et al.
Nature chemical biology, 15(7), 690-698 (2019-05-22)
Two-component systems (TCSs) are the largest family of multi-step signal transduction pathways and valuable sensors for synthetic biology. However, most TCSs remain uncharacterized or difficult to harness for applications. Major challenges are that many TCS output promoters are unknown, subject
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.