추천 제품
생물학적 소스
synthetic (organic)
Quality Level
분석
≥98% (HPLC)
양식
solid
색상
yellow
mp
168-170 °C
solubility
H2O: slightly soluble 0.17 mg/mL
45% (w/v) aq 2-hydroxypropyl-β-cyclodextrin: 2.8 mg/mL
0.1 M HCl: slightly soluble
DMSO: soluble
aqueous buffer pH > 5: soluble
ethanol: soluble
저장 온도
2-8°C
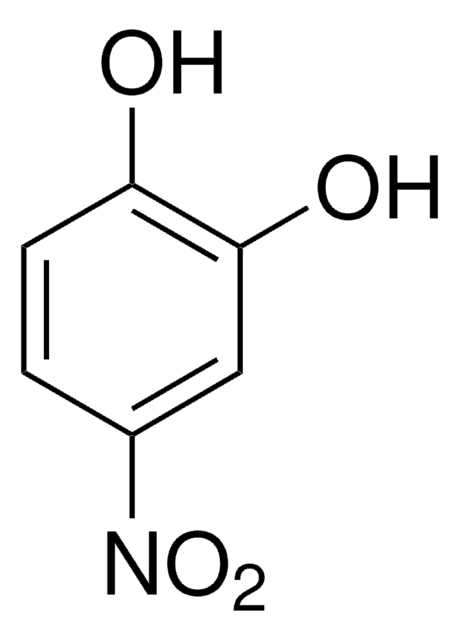
SMILES string
Oc1cc(cc(c1O)[N+]([O-])=O)[N+]([O-])=O
InChI
1S/C6H4N2O6/c9-5-2-3(7(11)12)1-4(6(5)10)8(13)14/h1-2,9-10H
InChI key
VDCDWNDTNSWDFJ-UHFFFAOYSA-N
유전자 정보
human ... COMT(1312)
애플리케이션
3,5-Dinitrocatechol (3,5-DNC) has been used in the preparation of the molybdenum (VI)-(3,5-DNC) complex. It has also been used as a catechol-O-methyltransferase (COMT) inhibitor and as a positive control for screening human COMT inhibition.
Chelating reagent used in a sensitive (μM) assay for vanadium.
생화학적/생리학적 작용
Selective inhibitor of catechol O-methyl transferase (COMT); penetrates the blood brain barrier and is useful both orally and parenteraly in experiments where inhibition of COMT in the central nervous system is required.
품질
Solutions may be stored for several days at 4 °C.
주의사항
Photosensitive
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
C Wiese et al.
Naunyn-Schmiedeberg's archives of pharmacology, 348(6), 582-585 (1993-12-01)
Organotypic primary cell cultures of fetal rat brain were used as a model system to study the effect of COMT inhibitors on the cerebral metabolic conversions of fluoro-DOPA enantiomers. The selective COMT inhibitors OR 486 and CGP 28014 were used
P T Männistö et al.
Life sciences, 43(18), 1465-1471 (1988-01-01)
Novel bisubstituted catechols were found to be potent and highly selective COMT inhibitors in vitro. One of them, OR-462 (3-(3,4-dihydroxy-5-benzylidene)-2,4-pentanedione), was studied also in vivo. When administered to rats orally together with levodopa and carbidopa, OR-462 greatly improved the bioavailability
O Kambur et al.
British journal of pharmacology, 161(7), 1553-1565 (2010-08-24)
Catechol-O-methyltransferase (COMT) inhibitors are used in Parkinson's disease in which pain is an important symptom. COMT polymorphisms modulate pain and opioid analgesia in humans. In rats, COMT inhibitors have been shown to be pro-nociceptive in acute pain models, but also
Raghvendra K Dubey et al.
Hypertension (Dallas, Tex. : 1979), 42(3), 349-355 (2003-08-13)
Local sequential conversion of estradiol to hydroxyestradiols and methoxyestradiols by CYP450 and catechol-O-methyltransferase, respectively, contributes to the antimitogenic effects of estradiol on glomerular mesangial cell growth via estrogen receptor-independent mechanisms. Catecholamines are also substrates for catechol-O-methyltransferase and therefore, might abrogate
Microplate screening assay to identify inhibitors of human catechol-O-methyltransferase.
Mika Kurkela et al.
Analytical biochemistry, 331(1), 198-200 (2004-07-13)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.