M1508
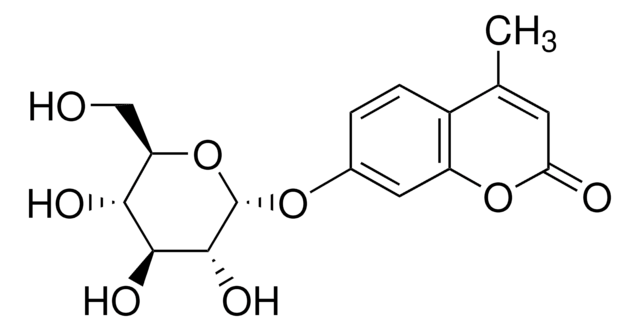
4-Methylumbelliferone sodium salt
fluorogenic, ≥98% (HPLC), crystalline
동의어(들):
β-Methylumbelliferone, 7-Hydroxy-4-methylcoumarin
About This Item
추천 제품
제품명
4-Methylumbelliferone sodium salt, ≥98% (HPLC), crystalline
Quality Level
분석
≥98% (HPLC)
양식
crystalline
색상
yellow
solubility
water: 50 mg/mL
SMILES string
[Na+].CC1=CC(=O)Oc2cc([O-])ccc12
InChI
1S/C10H8O3.Na/c1-6-4-10(12)13-9-5-7(11)2-3-8(6)9;/h2-5,11H,1H3;/q;+1/p-1
InChI key
JGMQHDNPUCPRQE-UHFFFAOYSA-M
일반 설명
애플리케이션
- as a substrate to analyze the enzyme kinetics of its glucuronidation in the human liver[2]
- as a standard to quantify the free 4-methylumbelliferone released as a result of enzyme-substrate action[3]
- in cell survival assay to inhibit hyaluronan (HA) production and to study its effect on chemoresistance in ovarian cancer[4]
생화학적/생리학적 작용
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.