P5396
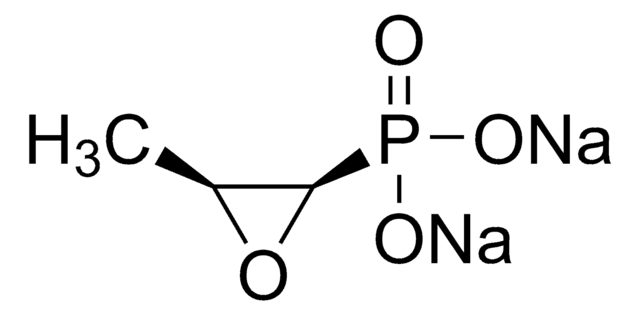
Phosphomycin disodium salt
antibacterial MurA inhibitor
동의어(들):
(−)-(1R,2S)-(1,2-Epoxypropyl)phosphonic acid, Fosfomycin, Phosphonomycin
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C3H5Na2O4P
Molecular Weight:
182.02
Beilstein:
4604425
EC Number:
MDL number:
UNSPSC 코드:
51102829
PubChem Substance ID:
NACRES:
NA.85
추천 제품
Quality Level
양식
powder
항생제 활성 스펙트럼
Gram-negative bacteria
Gram-positive bacteria
동작 모드
cell wall synthesis | interferes
저장 온도
2-8°C
SMILES string
[Na+].[Na+].C[C@@H]1O[C@@H]1P([O-])([O-])=O
InChI
1S/C3H7O4P.2Na/c1-2-3(7-2)8(4,5)6;;/h2-3H,1H3,(H2,4,5,6);;/q;2*+1/p-2/t2-,3+;;/m0../s1
InChI key
QZIQJIKUVJMTDG-JSTPYPERSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Phosphomycin, also known as Fosfomycin, is an antibiotic produced by Streptomyces fradiae. It is used to treat uncomplicated urinary tract infections. It is used for susceptibility studies of Klebsiella pneumoniae and to study in vitro susceptibility testing procedures for fosfomycin tromethamine.
생화학적/생리학적 작용
Antibiotic that concentrates in kidney and bladder; reduces nephrotoxicity and ototoxicity of platinum-containing anti-tumor agents. Fosfomycin is a phosphoenolpyruvate analog that irreversibly inhibits UDP-GlcNAc enolpyruvyl tranferase (MurA), an enzyme involved in bacterial cell wall biosynthesis. As a result, the production of N-acetylmuramic acid, an essential element of the peptidoglycan cell wall, is inhibited.
기타 정보
1g,5g,50g
Keep container tightly closed in a dry and well-ventilated place.Keep in a dry place.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
A L Barry et al.
Antimicrobial agents and chemotherapy, 35(6), 1235-1238 (1991-06-01)
Fosfomycin tromethamine (previously fosfomycin trometamol) is an orally administered fosfomycin which may be used for single-dose therapy of uncomplicated urinary tract infections. Fosfomycin tromethamine, norfloxacin, and trimethoprim-sulfamethoxazole inhibited greater than 90% of 352 bacterial isolates representing 25 different species; trimethoprim
Andrea Endimiani et al.
Antimicrobial agents and chemotherapy, 54(1), 526-529 (2009-11-11)
In vitro activity of fosfomycin was evaluated against 68 bla(KPC)-possessing Klebsiella pneumoniae (KpKPC) isolates, including 23 tigecycline- and/or colistin-nonsusceptible strains. By agar dilution, 93% of the overall KpKPC were susceptible (MIC(50/90) of 16/64 microg/ml, respectively). The subgroup of 23 tigecycline-
Nikos Fatsis-Kavalopoulos et al.
PLoS biology, 18(9), e3000856-e3000856 (2020-09-18)
Antibiotic combination therapies are important for the efficient treatment of many types of infections, including those caused by antibiotic-resistant pathogens. Combination treatment strategies are typically used under the assumption that synergies are conserved across species and strains, even though recent
Avigail Stokar-Avihail et al.
Cell host & microbe, 25(5), 746-755 (2019-05-10)
Temperate phages can adopt either a lytic or lysogenic lifestyle within their host bacteria. It was recently shown that Bacillus-subtilis-infecting phages of the SPbeta group utilize a peptide-based communication system called arbitrium to coordinate the lysogeny decision. The occurrence of peptide-based
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.