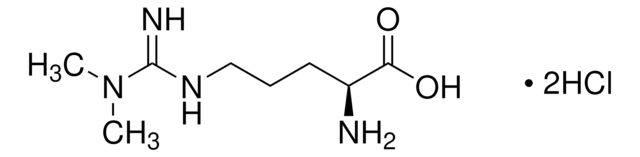
11009
L-Arginine
BioUltra, ≥99.5% (NT)
Synonym(s):
(S)-2-Amino-5-guanidinopentanoic acid
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(3)
Select a Size
Change View
About This Item
Linear Formula:
H2NC(=NH)NH(CH2)3CH(NH2)CO2H
CAS Number:
Molecular Weight:
174.20
Beilstein:
1725413
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
product line
BioUltra
Assay
≥99.5% (NT)
form
powder or crystals
optical activity
[α]20/D +27.0±0.5°, c = 5% in 5 M HCl
impurities
insoluble matter, passes filter test
≤0.3% foreign amino acids
ign. residue
≤0.05% (as SO4)
loss
≤0.1% loss on drying, 20 °C (HV)
color
white
pH
10.5-12.0 (25 °C, 0.5 M in H2O)
mp
222 °C (dec.) (lit.)
Looking for similar products? Visit Product Comparison Guide
Application
- L-Arginine-Dependent Nitric Oxide Production in the Blood of Patients with Type 2 Diabetes: A Pilot, Five-Year Prospective Study.: This study examines the role of L-Arginine in nitric oxide production in diabetic patients, proposing its potential benefits for managing vascular complications in diabetes (Stoian et al., 2024).
Biochem/physiol Actions
Substrate of nitric oxide synthase, which is converted to citrulline and nitric oxide (NO). Induces insulin release by a nitric oxide-dependent mechanism.
Other Notes
Exhibits stabilizing effects on plant protoplasts; pH drift correction in IEF of proteins; Growth requirement of various microorganisms.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A.W. Galston et al.
Plant Sci. Lett., 11, 69-69 (1978)
CRC Handbook of Microbiology, 4, 1-1 (1974)
H. Delice et al.
Electrophoresis '79, 165-165 (1980)
Krishnan Suresh Kumar et al.
European journal of medicinal chemistry, 45(11), 5474-5479 (2010-08-21)
A new series of 3-(benzylideneamino)-2-phenylquinazoline-4(3H)-ones were prepared through Schiff base formation of 3-amino-2-phenyl quinazoline-4(3)H-one with various substituted carbonyl compounds. Their chemical structures were elucidated by spectral studies. Cytotoxicity and antiviral activity were evaluated against herpes simplex virus-1 (KOS), herpes simplex
Palaniraja Thandapani et al.
Molecular cell, 50(5), 613-623 (2013-06-12)
Motifs rich in arginines and glycines were recognized several decades ago to play functional roles and were termed glycine-arginine-rich (GAR) domains and/or RGG boxes. We review here the evolving functions of the RGG box along with several sequence variations that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service