324716
Endo-α-N-acetylgalactosaminidase, Streptococcus pneumoniae, Recombinant, E. coli
Endo-α-N-acetylgalactosaminidase, Streptococcus pneumoniae, Recombinant, E. coli, CAS 59793-96-3, catalyzes the hydrolysis of the unsubstituted Galβ1,3GalNAc core disaccharide attached to Ser or Thr.
Synonym(s):
Endo-α-N-acetylgalactosaminidase, Streptococcus pneumoniae, Recombinant, E. coli, O-Glycopeptide endo-D-galactosyl-N-acetyl-α-galactosaminohydrolase, O-Glycosidase
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
conjugate
(O-linked)
form
liquid
specific activity
≥1 units/mL
≥10 units/mg protein
manufacturer/tradename
Calbiochem®
storage condition
do not freeze
foreign activity
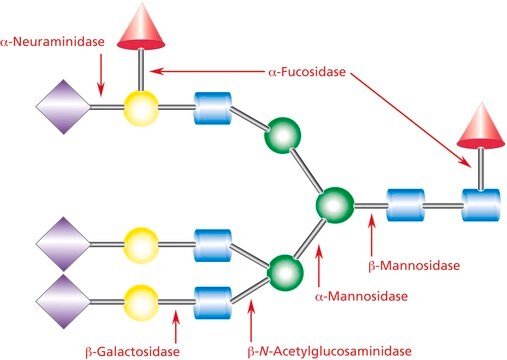
N-acetylglucosaminidase, α- and β-galactosidase, α-mannosidase, neuraminidase, proteases, none detected
shipped in
wet ice
storage temp.
2-8°C
Related Categories
General description
Warning
Unit Definition
Physical form
Other Notes
Iwase, H., and Hotta, K. 1993. Methods Mol. Biol. 14, 151.
Fan, J.Q., et al. 1990. Agric. Biol. Chem. 54, 233.
Umemoto, J., et al. 1978. Anal. Biochem. 91, 186.
Glasgow, L.R., et al. 1977. J. Biol. Chem. 252, 8615.
Legal Information
Storage Class Code
10 - Combustible liquids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Learn about O-linked glycan strategies, O-glycosidase actions, how to remove sialic acid residues, β-Elimination, and O-glycan modifications.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service