11080725001
Roche
Neuraminidase (Sialidase)
from Vibrio cholerae
Synonym(s):
Salidase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
Vibrio cholerae
Quality Level
form
solution
mol wt
~95 kDa
packaging
pkg of 1 U
manufacturer/tradename
Roche
optimum pH
5.5-6.2
suitability
suitable for ELISA applications
application(s)
life science and biopharma
sample preparation
shipped in
wet ice
storage temp.
2-8°C
General description
Approximately 40 U/mg enzyme protein at 37 °C and pH 5.5, with N-acetyl-neuraminosyl-D-lactose as the substrate.
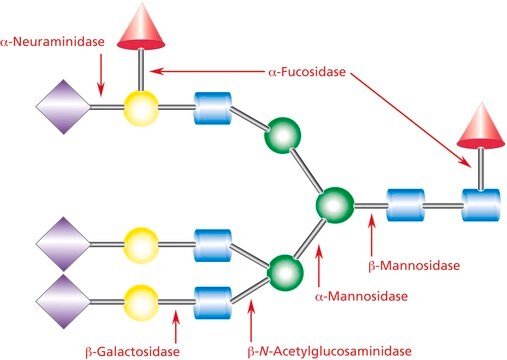
Neuraminidase is an acylneuraminyl hydrolase which hydrolyzes terminal N- or O-acylneuraminic acids which are α2,3-, α2,6-, or α2,8-linked (rate: α2,6 > α2,3 > α2,8) to oligosaccharides, polysaccharides, mucopolysaccharides, glycoproteins, and glycolipids. Noteworthy, for the hydrolysis of glycolipids, the presence of a detergent is necessary. Because of the broad substrate specificity, the enzyme is very well suited for the complete removal of sialic acids from glycoconjugates of a wide variety of biological materials (e.g., in cytology, on cell surfaces, viruses etc.).
Specificity
Hydrolyzes terminal N- or O-acyl-neuraminic acids that are α2,3-, α2,6-, or α2,8-linked to galactose, Hex, NAc, or N- or O-acylated neuraminyl residues in oligosaccharides/glycoconjugates or colominic acid. Relative rate of cleavage is α2,3 >α2,6 >α2,8, determined on bonds in tri- and tetrasaccharides.
Application
Neuraminidase has been used:
- to remove cis-acting sialic acids in CHO (chinese hamster ovary) cells
- for deglycosylation studies
Unit Definition
One unit is the enzyme activity that hydrolyzes 1 μmol N-acetyl-neuraminosyl-D-lactose within 1 min at +37 °C under the following incubation conditions:
10 mM N-acetyl-neuraminosyl-D-lactose, 50 mM sodium acetate, 4 mM calcium chloride, bovine serum albumin, 100 μg/ml, pH 5.5. The activity is determined by measuring the released D-lactose using the β-galactosidase/galactose dehydrogenase method. Under the same conditions, 1 μmol N-acetylneuraminic acid per min is split off from human acid α1-glycoprotein (10 mg/ml incubation mixture) by 1 U neuraminidase. Released N-acetyl-neuraminic acid can be determined using, for example, the thiobarbituric acid method.
10 mM N-acetyl-neuraminosyl-D-lactose, 50 mM sodium acetate, 4 mM calcium chloride, bovine serum albumin, 100 μg/ml, pH 5.5. The activity is determined by measuring the released D-lactose using the β-galactosidase/galactose dehydrogenase method. Under the same conditions, 1 μmol N-acetylneuraminic acid per min is split off from human acid α1-glycoprotein (10 mg/ml incubation mixture) by 1 U neuraminidase. Released N-acetyl-neuraminic acid can be determined using, for example, the thiobarbituric acid method.
Physical form
Solution in 50 mM sodium acetate, 154 mM sodium chloride, 9 mM calcium chloride, 0.1% Micr-O-Protect (w/v), human serum albumin, 25 mg/l, pH 5.5. The preparation contains 10 mM EDTA.
Note: The serum used for this preparation was tested for HBs antigen and for the presence of antibodies to HIV-1, HIV-2, HCV, and found to be negative, according to the current quality control procedures.
Note: The serum used for this preparation was tested for HBs antigen and for the presence of antibodies to HIV-1, HIV-2, HCV, and found to be negative, according to the current quality control procedures.
Other Notes
For life science research only. Not for use in diagnostic procedures.
Legal Information
The sale of the Product does not exhaust or grant any rights in third party patents including patents of companies of the F. Hoffmann - La Roche AG group of companies, in particular, for the use of modified antibodies obtained by using the product.
signalword
Warning
hcodes
Hazard Classifications
Skin Sens. 1
Storage Class
12 - Non Combustible Liquids
wgk_germany
WGK 1
flash_point_f
does not flash
flash_point_c
does not flash
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Angeles Canales et al.
JACS Au, 3(3), 868-878 (2023-04-04)
Influenza virus infection remains a threat to human health since viral hemagglutinins are constantly drifting, escaping infection and vaccine-induced antibody responses. Viral hemagglutinins from different viruses display variability in glycan recognition. In this context, recent H3N2 viruses have specificity for
Direct Attachment with Erythrocytes Augments Extracellular Growth of Pathogenic Mycobacteria.
Nishiuchi, et al.
Microbiology spectrum, 10, e0245421-e0245421 (2022)
Misako Nakayama et al.
Methods in molecular biology (Clifton, N.J.), 2556, 37-43 (2022-09-30)
Hemagglutinin (HA) on the surface of influenza viruses binds to sialic acids, mainly N-acetylneuraminic acid (Neu5Ac) or N-glycolylneuraminic acid. Neu5Ac and N-glycolylneuraminic acid lie at the terminal end of sugar chains on the cell surface. Human influenza viruses preferentially bind
Peter L Delputte et al.
Journal of virology, 81(17), 9546-9550 (2007-06-15)
The sialic acid-binding lectin sialoadhesin (Sn) is a macrophage-restricted receptor for porcine reproductive and respiratory syndrome virus (PRRSV). To investigate the importance of pSn sialic acid-binding activity for PRRSV infection, an R(116)-to-E mutation was introduced in the predicted sialic acid-binding
A previously uncharacterized O-glycopeptidase from Akkermansia muciniphila requires the Tn-antigen for cleavage of the peptide bond.
Medley, et al.
The Journal of biological chemistry, 298, 102439-102439 (2023)
Protocols
Neuraminidase can be used to cleave sialic acids from proteins. In this protocol, the enzyme from Vibrio cholerae is used on fixed cells.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service