M6882
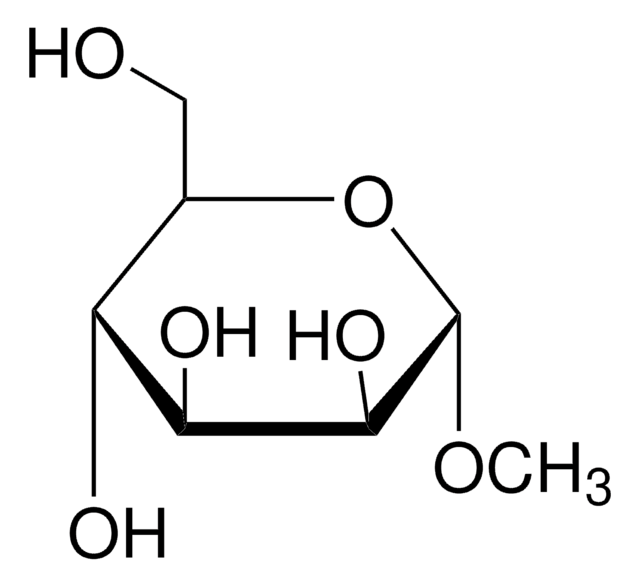
Methyl α-D-mannopyranoside
≥99.0% (HPLC)
Synonym(s):
α-Methyl D-mannoside
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Empirical Formula (Hill Notation):
C7H14O6
CAS Number:
Molecular Weight:
194.18
Beilstein:
81566
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
Quality Level
Assay
≥99.0% (HPLC)
form
powder
optical activity
[α]20/D 77.0 to 82.0°, c = 1-10% (w/v) in water
technique(s)
HPLC: suitable
color
white to off-white
mp
193-196 °C (lit.)
solubility
water: 100 mg/mL, clear, colorless
storage temp.
15-25°C
SMILES string
CO[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O
Looking for similar products? Visit Product Comparison Guide
General description
Methyl α-D-mannopyranoside is a competitor inhibitor of the binding of mannose by Escherichia coli.
Application
Methyl α-D-mannopyranoside has been used to synthesize a series of tri- and tetrahydroxylated seven-membered iminosugars in a study that worked towards a stable noeuromycin analog with a D-manno configuration. It has also been used in a study to investigate the primary mannose binding site of pradimicin A.
Other Notes
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
404.1 °F
Flash Point(C)
206.74 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yu Nakagawa et al.
Journal of the American Chemical Society, 133(43), 17485-17493 (2011-09-29)
Pradimicin A (PRM-A) is an actinomycete-derived antibiotic with the lectin-like property of being able to recognize D-mannopyranoside (Man) in the presence of Ca(2+) ion. PRM-A and its derivatives have been attracting a great deal of attention as the only family
M Aronson et al.
The Journal of infectious diseases, 139(3), 329-332 (1979-03-01)
Methyl alpha-D-mannopyranoside (alpha MM), a competitor inhibitor of the binding of mannose by Escherichia coli, was tested for its ability to prevent infection of the urinary tract of mice with infective strains of the organisms. Injection of the bacteria in
Karen T Welch et al.
Bioorganic & medicinal chemistry letters, 18(24), 6573-6575 (2008-11-08)
A virtual screening approach was used to identify new glycomimetics. The National Cancer Institute Diversity Set was docked into the carbohydrate binding site of the lectin concanavalin A (ConA). The resulting poses were analyzed and 19 molecules were tested for
Julia Deschamp et al.
Bioorganic & medicinal chemistry, 20(2), 641-649 (2010-10-26)
Noeuromycin is a highly potent albeit unstable glycosidase inhibitor due to its hemiaminal function. While stable D-gluco-like analogs have been reported, no data are available for D-manno-like structures. A series of tri- and tetrahydroxylated seven-membered iminosugars displaying either a D-manno-or
Justin D Mclaurin et al.
Molecular biology of the cell, 30(13), 1610-1620 (2019-05-02)
The Ras-Map kinase (MAPK) cascade underlies functional decisions in a wide range of cell types and organisms. In B-cells, positive feedback-driven Ras activation is the proposed source of the digital (all or none) MAPK responses following antigen stimulation. However, an
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service