T-039
Topiramate solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−20°C
SMILES string
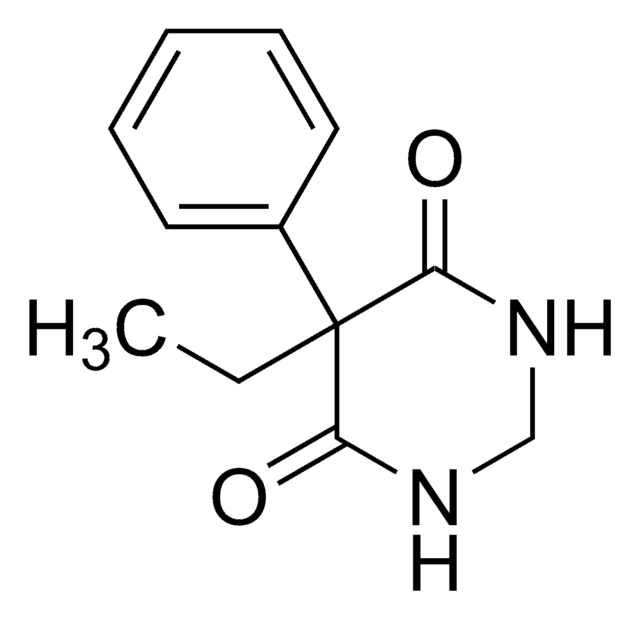
CC1(C)O[C@@H]2CO[C@@]3(COS(N)(=O)=O)OC(C)(C)O[C@H]3[C@@H]2O1
InChI
1S/C12H21NO8S/c1-10(2)18-7-5-16-12(6-17-22(13,14)15)9(8(7)19-10)20-11(3,4)21-12/h7-9H,5-6H2,1-4H3,(H2,13,14,15)/t7-,8-,9+,12+/m1/s1
InChI key
KJADKKWYZYXHBB-XBWDGYHZSA-N
Gene Information
human ... CA2(760) , CA4(762) , GABRA1(2554) , GABRA2(2555) , GABRA3(2556) , GABRA4(2557) , GABRA5(2558) , GABRA6(2559) , GABRB1(2560) , GABRB2(2561) , GABRB3(2562) , GABRD(2563) , GABRE(2564) , GABRG1(2565) , GABRG2(2566) , GABRG3(2567) , GABRP(2568) , GABRQ(55879) , GRIA1(2890) , GRIA2(2891) , GRIA3(2892) , GRIA4(2893) , GRIK1(2897) , GRIK2(2898) , GRIK3(2899) , GRIK4(2900) , GRIK5(2901) , SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
General description
Application
- Topiramate research solution: Research focuses on the development of intravenous formulations of topiramate for rapid intervention in seizure emergencies, highlighting its potential in emergency medicine and critical care (Epilepsy Behav, 2023; doi: 10.1016/j.yebeh.2023.109158).
- Topiramate liquid formulation: Innovations in drug delivery include the use of topiramate-chitosan nanoparticles, which offer enhanced drug stability and targeted delivery, particularly in addiction treatment settings (Neurochem Int, 2021; doi: 10.1016/j.neuint.2021.105157).
- Anticonvulsant biochemical reagent: Topiramate′s role as an anticonvulsant is further explored in studies focusing on its pharmacokinetic properties and interaction with other medications, emphasizing its importance in personalized medicine (Ther Drug Monit, 2019; doi: 10.1097/FTD.0000000000000600).
- Topiramate in vitro study: The compound is utilized in in vitro setups to investigate its effects on neuronal excitability, providing insights into its mechanism of action and potential therapeutic applications (Epilepsia, 2019; doi: 10.1111/epi.16073).
- Topiramate pharmacological research: Studies include the development of advanced analytical methods for the quantification of topiramate in biological samples, supporting its clinical monitoring and therapeutic drug management (J Pharm Biomed Anal, 2015; doi: 10.1016/j.jpba.2015.01.035).
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service