Chiral Chromatography Frequently Asked Questions
Read more about
- Chirality & Chiral Chromatography
- Chiral HPLC Column Selection & Screening
- Astec CHIROBIOTIC Chiral HPLC
- Astec CYCLOBOND Chiral HPLC
- Preparative Chiral HPLC
- Tips and Tricks for Chiral HPLC
- Chiral GC
Chirality & Chiral Chromatography
What is chirality?
Chirality belongs to the discipline of stereochemistry, which is the study of the 3-dimensional structure of molecules. Chiral compounds are optically active, that means they rotate polarized light to the left or to the right depending on their configuration. The word comes from the Greek stem “chir-” meaning hand, for handedness. Chiral molecules are like left and right hands – they are mirror images. With no amount of rotation can you make the two images or molecules overlap. A chiral compound will rotate the plane of polarized light; the degree to which it does this is called the specific rotation or optical rotation.
What are the types of isomers?
Isomers are molecules with the same chemical formula and often with the same kinds of bonds between atoms, but in which the atoms are arranged differently. The word comes from the Greek “isomeres” meaning having equal share. Isomers can be structural or spatial (stereoisomers). In structural isomers, the atoms and functional groups are joined together in different ways. For example, pentane can exist as three structural isomers: n-pentane, isopentane and neopentane; ortho-, meta- and para- substituted compounds are structural isomers as well. Stereoisomers (spatial isomers) are isomers of the same substance that only differ in the spatial arrangement of their atoms. The maximum number of stereoisomers that can exist for a compound is 2n, where n is the number of chiral centers. Chiral centers are atoms, usually C, bonded to four different groups. Stereoisomers that differ in the direction they rotate a plane of polarized light are called optically active, or chiral, and their isomers are called enantiomers. All enantiomers are stereoisomers but not all stereoisomers are enantiomers. Enantiomers rotate the plane of polarized light – one enantiomer to the left the other to the right. Enantiomers are non-superimposable mirror images. Diastereomer (or diastereoisomer) are molecules that possesses more than one chiral center. Diastereomers may be enantiomers if they are mirror images. If not, they are just diastereomers which DO differ in chemical properties and can be separated by conventional means. Anomers are diastereomers that differ only in the configuration of the first C atom. Epimers are diastereomers that differ only in the configuration of the second C atom. Meso compounds possess two chiral centers but are not optically active because rotation caused by one center is exactly offset by the rotation at the other center. There is a plane of symmetry through the center of the molecule; one half of molecule is the mirror image of the other.
What is a racemate?
Racemization is the loss of enantiomeric purity. Start with a single enantiomeric form, but end up with a mixture of enantiomers (racemate). Can be caused by increased temperature or biological conversion.
Racemic mixtures are mixture of (+) and (-) enantiomers in equal proportion. Given the +/-, R/S, d/l or D/L denotation depending on the type of compound. Racemic mixtures are optically inactive.
What nomenclature is used to distinguish the enantiomers?
(+) and (-) are experimentally verified by the direction the molecule rotates polarized light. (+) is clockwise rotation, (-) is counterclockwise rotation.
(R) and (S) (rectus and sinister) based on the direction of priority groups around the C, with the lowest priority group pointing away.
In (d) and (l), (D) and (L) The d or D stands for “dextro” meaning “right” and refers to clockwise rotation, the l or L stands for “levo” meaning “left” and refers to counterclockwise rotation. Amino acids, sugars and related compounds still refer to the D,L designation. This system, invented by Emil Fisher, refers to the configuration of the glyceraldehyde. He arbitrarily assigned the + isomer of glyceraldehyde as the D isomer. The d and l designation refers to the rotation of plane polarized light (sodium d-line). If this light is rotated to the right, the designation is “d” or (+). This assignment has problems as D-glutamic acid is actually l or (-) for the rotation of polarized light.Considerable care must be taken when using these symbols since they can only be related to each other after the actual rotation (+ or -) has been experimentally determined.
Why do companies care about the chiral purity of their compounds?
Besides the fact that one enantiomer is often safer and more efficacious than the other enantiomer, there are other arguments for having optically pure compounds. (1) Dosing is lower. If the product contains unwanted or inactive enantiomer, then they need to dose twice as much than they would if they had only the pure active enantiomer. (2) No interference of the desired activity by the unwanted enantiomer. In many cases, the unwanted enantiomer will have different biological activity and will interfere with the performance of the intended enantiomer. (3) Time savings in testing. If their product contains more than one enantiomer, they need to check the biological activity of each isomer plus the racemate to check for cooperative effects. This is three times the work than testing the pure enantiomer! These arguments are true for other industries besides pharmaceutical, for example agrochemicals. This has environmental implications as it can affect the total amount of chemical applied to the crop.
How are pure enantiomers obtained?
Since many compounds can exist as two or more enantiomers, how do chemists obtain a pure enantiomer? Generally there are two techniques that are employed: Synthetically and Post-synthetically. (1) Synthetically. Avoid racemic mixtures by using chirally pure starting materials (synthons), asymmetric synthesis, chemical catalysis or biocatalysis. (2) Post-synthetically using chromatography. Chiral stationary phases (HPLC and SFC) can separate racemic mixtures into the individual isomers and can be scaled-up to preparative sizes. The enantiomers can be converted into diastereomers using a chiral derivatization reagent or by using chiral mobile phase additives and then separated using conventional HPLC or GC. However this method is not often used in industry as it requires a chirally-pure derivatization reagent and the rates of reaction with each enantiomer have to be shown to be identical. If Simulating Moving Bed Prep (SMB) is possible, costs fall. New enzymatic (continuous) technologies are becoming available that convert the unwanted isomer back into the racemate for further prep. The most popular method of resolving racemic mixtures for process-scale operations is still the crystallization of diastereomeric salts.
What is the "e/e" ratio?
"e/e" refers to the ratio of one enantiomer to the other in a compound. For example, in prep chiral HPLC, the “pure” enantiomer may be 98% e/e.
How does chiral chromatography work?
Enantiomers of the same compound have identical physical properties. They only differ in: (1) the direction they rotate polarized light, (2) their biological activity (some), and (3) their interaction with other chiral molecules, like chiral stationary phases (CSPs) in chromatographic separations. Leveraging this last point to actually separate and quantify enantiomers is the function of chiral chromatography.
What detectors are used with chiral compounds?
HPLC detectors are available that are specific for detecting and measuring chiral compounds. These detectors (both in-line and off-line) are used to measure the enantiomeric content of the peak or the purified sample
Two types of chiral detectors are employed: Polarimeters and Circular dichroism spectrometers.
What is Polar Ionic Mode (PIM)?
This is a mobile phase system unique to CHIROBIOTIC CSPs. It is defined as a polar organic solvent (e.g. methanol) containing soluble salts, like ammonium acetate. A valuable feature of Astec CHIROBIOTIC CSPs, the novel and very versatile polar ionic mode is popular because its mobile phases are polar organic solvents containing volatile additives that are ideally suited for preparative and LC-MS applications. Compared to normal phase separations, the polar ionic mode has speed, efficiency, and sensitivity advantages, all valuable assets for LC-MS.
What is Polar Organic Mode (POM)?
Mobile phases that are a polar organic solvent or solvent blend. Enantiomers of polar neutral analytes have been successfully separated on Astec CHIROBIOTIC in the polar organic mode Reaction mixtures, even in pyridine, can be run on Astec CHIROBIOTIC in this mode.
Why is POM useful for chiral HPLC & LC/MS?
The benefit of POM is realized when dealing with compounds that are poorly soluble in non-polar normal phase mobile phases. For preparative chiral applications, solubility is especially important; analyte concentration per injection influences the throughput. POM mobile phases are also mass spec-friendly because they use methanol-based eluents with some acid and base or volatile ammonium salts as additives. As an additional sensitivity benefit, POM baselines are generally less noisy than normal phase baselines. Column and system equilibration in POM mobile phases is also very rapid, and there is no memory effect.
What types of CSPs can POM be used with?
The POM effect was first described by Armstrong in the early 1990's (1-3) on native and derivatized cyclodextrin-based CSPs (e.g. Astec CYCLOBOND). However, any CSP that can interact with solutes via hydrogen bonding mechanisms can utilize POM. These CSPs include the cyclodextrins, as well as macrocyclic glycopeptides (e.g. Astec CHIROBIOTIC), and polysaccharides (e.g. Astec Cellulose DMP).
Can POM be used with the popular cellulose-based CSPs?
Yes! Although the polysaccharide-based phases (cellulose and amylose) have proven their worth in normal phase and SFC systems, we have found they have good selectivity towards racemic compounds in POM mobile phases.
Is POM viable alternative to the normal phase?
Separations on POM can be more efficient, and provide more resolution than NP mode. Although this is very compound-dependent, POM should be explored if improvements in solubility, sensitivity, or instrument compatibility are desired. Figure 1 and Figure 2 show the antidepressant mianserin and Tröger's Base on Astec Cellulose DMP (a 3,5-diphenylcarbamate-deriviatized cellulose) in normal phase and POM systems. Note that in both cases, the POM separation gave comparable retention, but better resolution and sharper peaks.

Figure 1. Comparison of NP and POM on Astec Cellulose DMP (Mianserin) Mobile phase: See figure
Column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 µm particles (Product No. 51098AST)
Flow rate: 0.5 mL/min.
Det. UV at 230 nm
Sample: Mianserin, 2 mg/mL
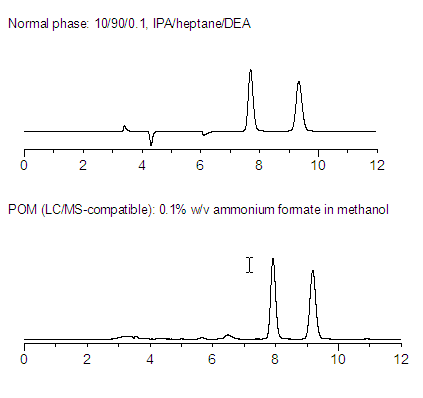
Figure 2. Comparison of NP and POM on Astec Cellulose DMP (Tröger's Base)
Column: Astec Cellulose DMP, 15 cm x 4.6 mm I.D., 5 µm particles (Produdct No. 51098AST)
Mobile phase: See figure
Flow rate: 0.5 mL/min.
Det. UV at 230 nm
Sample: Tröger's Base, 2 mg/mL
Chiral HPLC Column Selection & Screening
How do I choose a chiral column?
You can check to see if the separation appears in our applications database or has been published in the literature. Search our website, or contact our technical services group. If you have a novel compound, however, you will have to screen columns. You can check to see if there are published methods on structurally-related compounds, but structural similarity is no guarantee of similar behaviour on chiral stationary phases. Your most efficient approach is to call our chiral services group. We are fully equipped to do column screening, method optimization, and purification services. If you want to do the work yourself, choose whether HPLC, SFC, or GC. We can then guide you to the right set of columns to include in your screening protocol, and the systems to apply.
What type of approach should I take for screening chiral columns?
Even the experts have to screen columns. There is no reliable way to choose a chiral column based on analyte structure alone. Our Chiral Services Lab continually experiments with ways to improve the efficiency of their chiral column screening protocols. Currently a 94% success rate is achieved on a structurally-diverse, 32-compound screening set (Table 1). The description of our screening protocol and success or "hit" rates are shown in Table 2. We use two HPLC instruments, one dedicated to normal phase (NP) operation and the other to reversed-phase (RP), polar ionic mode (PIM), and polar organic mode (POM). Polysaccharide and synthetic polymer CSPs are screened in NP and POM systems. Astec CYCLOBOND CSPs are screened in POM and RP. Astec CHIROBIOTIC CSPs are also screened in RP mode, but are most useful in PIM. PIM is unique to CHIROBIOTIC and very valuable for enantioresolution of polar and ionic compounds. When we encounter analytes with a primary amine moiety (especially one at or near the chiral center), we use a third NP and a third POM mobile system and screen three cyclofructan CSPs. All of the columns in our screen are very rugged, and do not have to be dedicated to operation in a single mobile phase system. This brief description of our screening protocol is meant to demonstrate two points. First, stationary phase selectivity is your biggest lever to affect enantioresolution. Second, having a wide range of mobile phase options, from highly polar ionic and aqueous to nonpolar systems, gives you flexibility with detectors and a means to increase analyte solubility. The latter is a very important consideration for prep chiral separations.
Do you have any application database to search for columns and conditions?
Yes! We are continually adding to the chiral applications on our website. Usually a Google search will pull up our applications. But you can go to our website, type in the name of the compound in the search bar (add "enantiomer" to narrow the search). If we have the application, then it will appear on the "Analytical Application" tab. You can also search our chiral bibliography where we try to maintain an up-to-date listing of where our columns have been cited in the scientific literature. Or, visit sigma-aldrich.com/applications-search.
Can we select a column for our application based on a published reference for another analyte with a similar functional group?
Structural similarity is no guarantee of similar behaviour on chiral stationary phases. If you can't find an exact compound match, you will have to screen columns, or call our chiral services group. Our chiral method development wallchart does give some guidelines on optimizing methods if you have acidic, basic, or neutal analytes.
What specific column and mobile phase will separate a particular set of enantiomers?
People think that chiral chemistry is cut and dry and that we should be able to just look at a compound and tell them what would give enantiomeric selectivity. Unfortunately, as you know, that usually isn't the case. We typically recommend a chiral screen if we don't have any applications on that particular analyte.
Which is the best column in your entire chiral list, which could be tried for the multiple applications?
For HPLC, we recommend a cellulose column (Astec Cellulose DMP or Kromasil CelluCoat), Astec CHIROBIOTIC V2, T, and TAG. These 4 CSPs will separate the vast majority of enantiomers under different mobile phase conditions. For GC, we recommend Astec CHIRALDEX G-TA, G-DP, B-DM, and Supelco ß-Dex 110 or 120.
What is your column that is equivalent to {a specific competitive column}?
Unfortunately, it rarely works out that a chiral columns are interchangeable. Chiral retention mechanisms are complex.
Do you have any equivalent column which will match Daicel columns?
All chiral columns are different. However, in selectivity we offer the Astec Cellulose DMP and Kromasil® CelluCoat™ (available in US & Canada only) which perform similarly to the CHIRALCEL® OD-H and phases based on the DMPC-derivatized cellulose technology.
For CHIROBIOTIC and CYCLOBOND (and any of our columns) how can I successfully add these columns to my current column screen, with conditions for quick, easy method development?
We offer a Method Development chart that shows easily and clearly how to incorporate the Astec CSPs into your screening protocol, and how to develop and optimize methods. See the FAQ sections on CHIROBIOTIC and CYCLOBOND for tips on method development and optimization on these phases.
I am interested in adding your columns to my chiral screens, but how can I do that if my system is set up for normal phase, not reversed-phase?
For normal phase and SFC, include our cellulose-based columns (Astec Cellulose DMP, Kromasil Cellucoat), Astec P-CAP and P-CAP-DP, and Astec CYCLOBOND I 2000 DMP and DNP. Also consider the benefits of polar ionic mode (PIM) and reversed-phase modes for polar/ionic compounds, and to optimize analyte solubility and LC-MS compatibility.
What columns do you recommend for chiral SFC (Supercritical Fluid Chromatography)?
Any column that can be run in normal phase can be run in SFC. For normal phase and SFC, include our cellulose-based columns (Astec Cellulose DMP, Kromasil Cellucoat), Astec P-CAP and P-CAP-DP, and Astec CYCLOBOND I 2000 DMP and DNP.
Astec CHIROBIOTIC Chiral HPLC
What is the Astec CHIROBIOTIC family?
Invented by Prof. Daniel W. Armstrong, the Astec CHIROBIOTIC family comprises highly enantioselective CSPs based on macrocyclic glycopeptides that have been bonded through multiple covalent linkages to high purity silica particles. Astec CHIROBIOTIC CSPs offer flexibility in choice of mobile phase conditions, both aqueous and nonaqueous, and are ideal for analytical and preparative separations of neutral, polar and ionic compounds.
How do Astec CHIROBIOTIC CSPs separate enantiomers?
Astec CHIROBIOTIC CSPs offer six different types of molecular interactions: ionic, H-bond, π-π, dipole, hydrophobic, and steric. They also possess multiple inclusion sites that influence selectivity based on the molecular shape of the analyte. The optimization of enantiomer resolution is achieved by changing the mobile phase to leverage the types and relative strengths of the various interactions.
How do the Astec CHIROBIOTIC CSPs differ?
The various Astec CHIROBIOTIC phases share the benefits of robustness, flexibility in mobile phase options, ionic interactions, compatibility with polar compounds and LC-MS, and preparative scalability. However, Astec CHIROBIOTIC CSPs differ in selectivity, primarily because of their differing number and types of interaction sites, and the number, type, and accessibility of ionic sites in the bonded macrocyclic glycopeptide.
What makes Astec CHIROBIOTIC CSPs unique?
The bonded macrocyclic glycopeptide itself, in terms of its morphology, molecular composition, and multiple covalent linkages to the silica surface, is what makes Astec CHIROBIOTIC CSPs unique and gives them significant and valuable benefits over other CSPs. The truly differentiating feature of Astec CHIROBIOTIC CSPs is the presence of ionic interactions. These interactions are unique to Astec CHIROBIOTIC CSPs and are responsible in large part for their desirable retention characteristics toward polar and ionizable analytes in aqueous and non-aqueous solvents.
What is the difference between the CHIROBIOTIC CSPs?
CHIROBIOTIC CSPs are based on different macrocyclic glycopeptides (natural products of microbial origin). Presently, we offer 4 different ones: teicoplanin, vancomycin, ristocetin, and teicoplanin aglycone. Each one is unique in the types of molecular interactions it provides, and the number of chiral centers. They give very different enantioselectivity, but they all offer the benefit of ionic interactions and ability to operate in aqueous reversed-phase, polar ionic, and polar organic mobile phase systems.
What is the difference between the CHIROBIOTIC V and V2; T and T2?
Astec CHIROBIOTIC V and T differ from V2 and T2, respectively, in their bonding chemistry that gives them different selectivity and preparative capacity for certain classes of analytes.
What are the recommended CHIROBIOTIC screening protocols?
Polar ionic (PIM) - methanol/acetic acid/TEA (100:0.1:0.1 v/v/v). Optimize by changing acid-base ratio (up to 1% of each), change the type of acid or base, or adding a volatile salt (test different ammonium salts).
Reversed-phase (RP) - methanol or acetonitrile/20 mM ammonium acetate, pH 5 (30:70). Optimize by changing % or type of organic modifier, adjust pH, buffer type, ionic strength.
Polar organic (POM) - ethanol. Optimize by using other polar organic solvents or blends.
Normal phase (NP) - ethanol/heptane (30:70). Optimize by changing % of polar modifier, or change both solvents.
What is the effect of different salts in PIM?
In polar ionic mode (PIM), DEA or ammonium hydroxide can replace TEA, but selectivity will be different
How do I use the acid-base ratio to adjust enantioselectivity and retention?
CHIROBIOTIC CSPs possess ionic functional groups and are ideal for use in the so-called polar ionic mode, which is defined as polar organic solvents (methanol or acetonitrile) to which are added soluble salts, acids, bases or combinations of these additives. The polar ionic mode is useful for ionizable compounds, where a good starting mobile phase is 0.1% TEA/ 0.1 % acetic acid in methanol. In the case of LC-MS, use volatile ammonium salts, like ammonium formate or ammonium trifluoroacetate. When working with polar ionic mode, method development and optimization involves adjusting the ratio of acid (typically acetic acid) to base (typically TEA) to adjust selectivity and retention. The ratio depends on the pKa of the enantiomers and other factors. For basic analytes, use higher proportion of acid, while for acid analytes, use higher proportion of base. The acid-base ratio can be from 4:1 to 1:4. A 1:1 ratio is used for screening purposes. If the retention is still too short, using a low concentration of TEAA (0.1%) or replacing the methanol with up to 50% acetonitrile will reduce the mobile phase strength. However, if the compound is neutral and not very polar, there will be little or no retention under polar ionic conditions and a different mobile phase system, like normal phase, should be tried.
When is it advisable to add water to mobile phases in polar ionic mode (PIM)?
The ionic functional groups present in CHIROBIOTIC phases means you can use polar additives (salts, water) to improve method development, method reproducibility and column performance. When developing a method for ionizable analytes (acids, bases, zwitterions) on Astec CHIROBIOTIC columns, the polar ionic mode (PIM) is a good starting point. The polar ionic mode mobile phase is methanol containing 0.1% w/v ammonium formate. Vary the NH4OH:formic acid ratio, or try other ammonium salts (e.g. trifluoroacetate or acetate) for optimization. In LC-MS applications, a small amount of water (3-5%) can be added to the mobile phase to improve sensitivity and reproducibility of the method. The addition of water helps to improve the solubility of salts in the methanol.
How do I optimize my chiral separation for LC/MS?
For CHIROBIOTIC separations in PIM mode use volatile salts (e.g. ammonium acetate, ammonium formate, ammonium TFA). For CYCLOBOND separations in POM mode, replace the TEA with ammonium hydroxide, lower the concentration by 50-75%. For both types of columns in reversed-phase (RP) mode, use ammonium acetate or ammonium formate.
How can I improve the ruggedness and repeatability of a polar ionic (PIM) method?
Resolution of polar and ionic enantiomers is a unique and valuable feature of Astec CHIROBIOTIC CSPs. The mobile phases for these separations are quite simple: polar organic solvents, such as methanol or acetonitrile, containing volatile acids and bases or their corresponding salts. The concentrations of the acid and base or the acid:base ratio are varied to optimize retention and selectivity. However, retention time variation and slow equilibration may occur when these additives are employed at high concentrations or when the solubility of the additive in the solvent is limited. We have found that the addition of a small amount of water to the mobile phase increases the additive’s solubility, reduces equilibration time and improves the stability of retention times if needed. Typically, the addition of 5 to 10% v/v water to the methanol mobile phase was sufficient to provide stable retention and maintain enantioselectivity, while also maintaining the same ionic mechanism.
How can I stabilize the retention in polar ionic mode (PIM) separations?
Adding 5% water to methanol/0.1% ATFA is important for some PIM applications if the retention time is not as stable as it should be.
How do I recondition my CHIROBIOTIC column?
The ionic functional groups present in CHIROBIOTIC phases means you can use polar additives (salts, water) to improve method development, method reproducibility and column performance. To keep your columns in top performance and maximize lifetime and method reproducibility during operation, macrocyclic antibiotic-based Astec CHIROBIOTIC columns should be cleaned once a week with 20 column volumes of a (50:50) acetonitrile:50 mM ammonium acetate mobile phase, followed by 20 column volumes of 100% methanol. Before storing the column, we recommend that you run a quick QA test using 5-methyl-5-phenylhydantoin with methanol as the mobile phase. Refer to the QA test report that is included with the column for full details.
How do I retest CHIROBIOTIC columns?
To ensure the selectivity performance of CHIROBIOTIC columns, periodically test with 5-methyl-5-phenylhydantoin column in 100% methanol mobile phase.
What is the best storage solvent for CHIROBIOTIC columns?
Acetonitrile or methanol are suitable for short term storage, for longer-term storage (>24 hours) isopropanol is recommended.
How do I determine which column is best for separating Amino Acids?
Most amino acids are chiral, with the L-form dominating in nature. D-Amino acids and the ratio of D to L-forms are studied for various reasons, including pharmaceutical research and to explore their extraterrestrial origins. Astec chiral HPLC and GC stationary phases are uniquely suited for chiral amino acid separations, including native amino acids and their N-blocked derivatives. CHIROBIOTIC CSPs have ionic functional groups and therefore permit chiral discrimination of compounds with ionic or ionizable groups. They also operate in mobile phases that are MS-compatible and in which amino acids are freely soluble. Other phases, notably Astec CLC and various CYCLBOND chemistries, also have found utility for amino acid enantiomer separations. See our decision tree for a general guideline on choosing an HPLC CSP for chiral amino acid separations.

Astec CYCLOBOND Chiral HPLC
What is CYCLOBOND?
The versatile and unique Astec CYCLOBOND™ CSPs (chiral stationary phases) are a family of derivatized and underivatized β- and γ-cyclodextrins bonded to high-purity silica gel. Invented by Professor Daniel W. Armstrong and introduced to the market in 1984, they have found widespread use for isomer separations by HPLC, both chiral and achiral. Astec CYCLOBOND is complementary to other CSPs, including the polysaccharide-based CSPs, macrocyclic glycopeptide-based Astec CHIROBIOTIC® CSPs, and the amine copolymer-based Astec P-CAP™ and Astec P-CAP-DP.
What are cyclodextrins?
Cyclodextrins are produced by partial degradation of starch, followed by the enzymatic coupling of glucose units into crystalline, homogeneous toroidal structures of different molecular size. The D(+)-glucose residues are bonded to each other through a-(1,4)glycosidic linkages. The chair configuration of glucose residues makes the toroid “bucket” narrower at one end. Three highly-characterized cyclodextrins are alpha (α), beta (ß), and gamma (γ) cyclodextrin, which contain six, seven, and eight glucose units, respectively. Because each glucose residue has five chiral centers, cyclodextrins are themselves chiral structures. For example, ß-cyclodextrin has 35 chiral centers.
How do cyclodextrin-based CSPs (including CYCLOBOND) separate enantiomers?
Both the architecture and chemistry of cyclodextrins contribute to enantiomer separations. The toroidal cyclodextrin structure has a hydrophilic exterior surface resulting from the 2-, 3-, and 6-position hydroxyl (OH) groups. The interior cyclodextrin cavity is composed of the glucose oxygens and methylene hydrogens, which gives it a non-polar (hydrophobic) character. Chemical interactions that lead to chiral separations occur on both the exterior and interior surfaces of the cyclodextrin toroid. The most important consideration for retention and chiral recognition is proper fit of the analyte into the cyclodextrin cavity. This fit is a function of both molecular size and shape of the analyte relative to the cyclodextrin cavity. Thus, there are two basic mechanisms at play in chiral separations on cyclodextrins: those that occur on the inside cavity surface (inclusion complexing) and those that occur on the outside surface (surface interactions) of the cyclodextrin toroid.
What is "inclusion complexing"?
The basis for many separations on cyclodextrin-based CSPs in the reversed-phase mode (mobile phases containing water with methanol or acetonitrile) is a phenomenon called inclusion complexing. If the analyte can fit into the cyclodextrin cavity and mobile phase conditions are favorable, the inclusion complexing mechanism can occur. It is because of inclusion complexing that reversed-phase is a very successful mode on CYCLOBOND CSPs. Three points of interaction are required for a chiral discrimination, and the inclusion complexing provides one of the three interactions. The inclusion complexing mechanism is attributed to the attraction of the apolar molecule or segment of the molecule to the apolar cyclodextrin cavity, which is very sensitive to structural differences. When the analyte possesses an aromatic group, the orientation in the cavity is selective due to the sharing of electrons between the aromatic methylene groups and the glucoside oxygens on the internal surface of the cyclodextrin toroid. The mechanism is completed by interaction of solute functional groups with the 2- and 3- position secondary hydroxyl groups of the cyclodextrin ring.
What makes Astec CYCLOBOND CSPs unique?
CYCLOBOND CSPs offer unique chiral selectivity by way of multiple chiral mechanisms provided by the cyclodextrin cavity and the functional groups of the various derivatives. CYCLOBOND CSPs feature chemical stability for long lifetime, wide mobile phase choices, and high efficiency.
What types of enantiomers are separated on Astec CYCLOBOND CSPs?
In general, substituted phenyl, naphthyl, and biphenyl rings can be separated on ß-cyclodextrin-based CYCLOBOND I 2000 and its derivatives. Molecules with heterocyclic rings also often separate on these phases. Analytes with three to five rings, including steroids, are best separated on g-cyclodextrin-based CYCLOBOND II and its derivatives. Enantiomers with halogens, nitrates, sulfates, phosphates, and hydroxyls on the analyte’s aromatic rings generally separate well on CYCLOBOND CSPs. Also successfully resolved on CYCLOBOND are compounds with hydrogen-bonding functional groups off a ring, cis/trans and positional isomers closely-related achiral molecules, and derivatized chiral amino acids.
What are the recommended CYCLOBOND screening protocols?
Reversed-phase (RP) - acetonitrile/20 mM ammonium acetate, pH 5 (30:70) or methanol/20 mM ammonium acetate, pH 5 (20:80). Optimize by changing % or type of organic modifier, adjust pH, buffer type, ionic strength.
Polar organic (POM) - acetonitrile/methanol/acetic acid/TEA (95:5:0.1:0.1). Optimize by using other polar organic solvents or blends. Change acid-base ratio (up to 1% of each). Note that methanol and acetonitrile show large differences on CYCLOBOND in POM mode.
Normal phase (NP) - ethanol/heptane (30:70). Optimize by changing % of polar modifier, or change both solvents.
How do I recondition my CYCLOBOND column?
Flush columns with 10 column volumes each of ethanol then HPLC-grade water. Then flush with 10 column volumes of acetonitrile at a low flow rate. The ethanol is two times more efficient for displacing substances from the cavity than methanol.
What is the best storage solvent for CYCLOBOND columns?
Acetonitrile is suitable for short term storage, for longer term storage (>24 hours) isopropanol is recommended.
How do I retest CYCLOBOND columns?
Refer to the column QA report, consult the instructions for CYCLOBOND or contact Technical Services for the test procedure.
Preparative Chiral HPLC
What about preparative isolations on Astec chiral HPLC columns?
A significant advantage of Astec CHIROBIOTIC for preparative applications is the fact that the mobile phase can be chosen to optimize sample solubility – a critical preparative consideration. Astec CHIROBIOTIC columns can be used in all preparative HPLC techniques, including elution and recycle chromatography, mass directed prep, SFC, and simulated moving bed (SMB). Scale-up is highly predictable because the same bonded phase chemistry is employed across all particle sizes. Multiple covalent bonds attach the Astec CHIROBIOTIC macrocyclic glycopeptides to the silica surface, meaning no CSP ligand will contaminate the product. Preparative separations on Astec CHIROBIOTIC columns often have speed and efficiency benefits over other CSPs. In terms of loading capacity, a 25 cm x 21.2 mm column has medium to high loadings, from a few mg to over 300 mg per injection. Preparative separations on Astec CHIROBIOTIC are reproducible and scalable.
What is SMB for prep?
SMB (simulated moving bed) is a form of preparative chromatography that utilizes multiple columns that act in concert as a single column. Throughput using SMB can be significantly higher than batch or column format. The qualities of Astec CHIROBIOTIC CSPs that make them ideal for prep, including SMB, are excellent enantioselectivity, especially for polar and ionic compounds, mobile phase flexibility to maximize sample solubility, versatility for operation in all mobile phases without memory effects and high column efficiency for high throughput and minimal downstream processing. Especially relevant for prep by SMB, the ruggedness of CHIROBIOTIC CSPs enables long-term and reliable operation. The new Astec CHIROBIOTIC columns for SMB feature particle size and column dimensions chosen for high flow rate and high efficiency. The set's 8 columns have efficiencies that are matched to within 6% rsd. Single columns in 25 cm x 4.6 mm I.D. and 5 cm x 10 mm I.D. dimensions are available for method development and scale-up experiments.
Tips and Tricks for Chiral HPLC
Are there any flow rate considerations in chiral HPLC?
Because most chiral stationary phases are very complex (densely bonded, large molecules), they suffer from high C term in the van Deemter equation. This is high resistance to stationary phase mass transfer. Therefore, above the optimum flow rate, the efficiency drops off very quickly. For critical separations where the enantiomers are not well separated, it is worth studying the effect of flow rate on resolution for your separation. Van Deemter studies performed on 4.6 mm I.D., 5 µm particle CHIROBIOTIC T and V2 columns in reversed-phase, polar-ionic, and polar-organic modes have shown that the greatest peak efficiency is achieved at flow rates between 0.15 and 0.2 mL/min. (linear velocities between 0.2 and 0.3 mm/s; reduced linear velocities between 0.61 and 0.81). Therefore, decreasing the flow rate beyond the norm may enhance the efficiency of each peak, and thus, improve the resolution of the enantiomers. Once enantiomeric selectivity is achieved on a chiral stationary phase, resolution of the enantiomers may be improved by changing mobile phase composition, temperature, and/or flow rate. A flow rate of 1.0 mL/min. is often used during method development on 4.6 mm I.D. columns packed with 5 µm particles. For method optimization, if enantiomers are separated but not fully resolved at 1 mL/min., decreasing the flow below 1.0 mL/min. may further increase resolution.
What is the impact of temperature in chiral HPLC?
Temperature can be leveraged to change the enantioselectivity of a separation on chiral stationary phases. The role of temperature is a very complex thermodynamic relationship between solute/mobile phase/CSP interactions. The change of temperature will change the thermodynamic behavior of solutes resulting from above-mentioned interactions. Sometimes increasing the temperature improves resolution, while sometimes decreasing it gives the desired improvement. This phenomenon is unpredictable, thus temperature gives chromatographers another tool for optimization.
How do I measure the void volume of a chiral HPLC column?
Because of the many different types of interactions possible with most chiral stationary phases, it is quite difficult to find a totally unretained compound. For an HPLC column packed with traditional porous spherical silica particles, a rough estimate of the void volume is about 70% of the column volume (0.7πr2L). For a 25 cm x 4.6 mm column, this would be about 3 mL. At 1 mL/min. this would give a T0 of 3 min. at 1 mL/min.
Can I use gradient elution in chiral HPLC, and when should I try it?
HPLC phases where the chiral ligand is covalently bonded to the particle surface, like Astec CHIROBIOTIC, Astec CYCLOBOND, and Astec P-CAP and P-CAP-DP, are fully compatible with gradient elution. But when would one use gradient elution in chiral HPLC? First, gradients can be used to speed up the screening process with a more generic method that would cover a wider range of sample types. Selectivity detected with gradients could then be optimized for isocratic. The Astec CHIROBIOTIC and most CYCLOBOND phases that are run in reversed-phase mode and can utilize typical RP-type gradients (increasing solvent strength by increasing % of organic modifier). Additionally, because of the ionic character of Astec CHIROBIOTIC, they also respond to increasing ionic strength (increasing % of soluble salt additive, e.g. ammonium acetate, 0 - 0.2%). The second reason for using gradients is to maintain cleaner, uncontaminated columns when impure samples are injected. Gradients can be run to remove strongly-bound matrix components from the columns. (Note the regeneration/reconditioning process for CHIROBIOTIC phases to restore ionic character recommended in the FAQ: "How do I recondition my CHIROBIOTIC column?". For more discussion on whether gradient elution would be appropriate for your samples and system, please consult our technical services.
How do you recommend converting instruments from normal phase (NP) to reversed-phase (RP) and back?
The best solution is to have dedicated instrument to each mode. There is significant wear-and-tear on the seals caused by expansion/contraction. However, if you can't here are some things we found important:
(1) Our regular practice is to flush extensively with IPA before going over to water. In all the flushing, be sure to include the entire fluid path (pump, autosampler, detector).
(2) As for the autosampler, be sure to include the sample loop and any other fluid paths that are encountered in it's normal operation of making injections -- this can vary a lot depending on whether the autosampler is an external loop design, or an internal loop design.
(3) As part of all the washes, make sure the injection needle gets washed as well -- maybe do several full loop injections of the solvent (no column, of course).
(4) It may also be a good idea to flush with methanol (or ethanol) after the IPA before going to water. Methanol will help flush the IPA out faster than going directly from IPA to water. This all of course, presumes that the IPA is miscible with the weakest NP solvent the system has been exposed to.
(5) So going the other way (RP-> NP) is just the reverse.
Converting RP to NP and vice versa (very important to add some don’ts with the dos):
· Do remove all additives and start with 100% isopropanol in all reservoirs.
· Isopropanol is fully miscible with all common solvents and is the safest changeover solvent for either direction.
· Do use low flow- about half of normal to avoid possible column damage due to over-pressuring.
· Don’t use acetonitrile routinely as the organic- it is better than methanol, but is not fully miscible with pure hydrocarbons.
· Don’t use methanol routinely as the organic- it is not fully miscible with many normal phase conditions.
· Either acetonitrile or methanol may be used to routinely change from RP to HILIC (NP of polar solutes using polar aqueous mobile phases) and back.
· Do check miscibility (use small external vessel) with target mobile phase before starting, especially if isopropanol is not selected.
· If changing over a gradient instrument, use organic in all lines to make certain that water or hydrocarbon is removed from all fluid areas.
· Do monitor pressure and detector signals during changeover as excellent methods to confirm full system equilibration.
· Incomplete mixing shows up as severe detector baseline noise or pressure fluctuations (globules of immiscible solvent resembles bubbles).
· Do flush detectors and all other components even if baseline is not monitored.
· Do check gradient blank runs for baseline noise that might indicate pockets of immiscible solvent.
· Good chromatography is the final test- use simple standard test mixes first and work toward real samples.
· Total time for changeover can vary but should take about an hour- rushing usually slows down the process.
· Don’t expect fast changeover with refractive index detectors- they are extremely slow to equilibrate after changeover.
What are some guidelines for chiral trace analysis?
Using 5% 20mM ammonium phosphate (pH 3.1)/95% methanol can be very useful for trace analysis and for compounds with both COOH and NH near C*. You also should consider the elution order. The enantiomer in trace amounts should elute first. Consider also narrow I.D. columns (2.1 mm or 1 mm). Anything that will increase the efficiency will also increase sensitivity. Generally, the biggest lever is flow rate: make sure you are operating at the optimum flow rate for the column dimension. In chiral HPLC, the optimum flow rate can be very slow.
How do I optimize my chiral separation for LC/MS?
For CHIROBIOTIC separations in PIM mode use volatile salts (e.g. ammonium acetate, ammonium formate, ammonium TFA). For CYCLOBOND separations in POM mode, replace the TEA with ammonium hydroxide, lower the concentration by 50-75%. For both types of columns in reversed-phase (RP) mode, use ammonium acetate or ammonium formate.
How can I remove phospholipid interference in bioanalytical chiral LC-MS?
Astec CHIROBIOTIC columns can be used in conjunction with HybridSPE™-PPT plates to enhance sensitivity by completely removing endogenous proteins and phospholipids.
How do I make up TEAA buffer?
A 0.1% TEAA (triethylamine acetate) solution is often used in reversed phase (RP) and polar ionic mode (PIM) mobile phases. For RP, add the TEA v/v in water, titrate to the desired pH with acetic acid. For PIM mobile phases we use 100/0.1/0.1, methanol/acetic acid/TEA (v/v/v), which is made by adding 1 mL TEA and 1 mL acetic acid to 1 L of methanol.
How do I stabilize retention times in polar ionic mode (PIM)?
Adding 5% water to methanol/0.1% ATFA is important for some PIM applications if the retention time is not as stable as it should be
How can I improve the ruggedness and repeatability of a polar ionic (PIM) method?
Resolution of polar and ionic enantiomers is a unique and valuable feature of Astec CHIROBIOTIC CSPs. The mobile phases for these separations are quite simple: polar organic solvents, such as methanol or acetonitrile, containing volatile acids and bases or their corresponding salts. The concentrations of the acid and base or the acid:base ratio are varied to optimize retention and selectivity. However, retention time variation and slow equilibration may occur when these additives are employed at high concentrations or when the solubility of the additive in the solvent is limited. We have found that the addition of a small amount of water to the mobile phase increases the additive’s solubility, reduces equilibration time and improves the stability of retention times if needed. Typically, the addition of 5 to 10% v/v water to the methanol mobile phase was sufficient to provide stable retention and maintain enantioselectivity, while also maintaining the same ionic mechanism.
How can I stabilize the retention in polar ionic mode (PIM) separations?
Adding 5% water to methanol/0.1% ATFA is important for some PIM applications if the retention time is not as stable as it should be.
How do I using the acid-base ratio to adjust enantioselectivity and retention?
CHIROBIOTIC CSPs possess ionic functional groups and are ideal for use in the so-called polar ionic mode, which is defined as polar organic solvents (methanol or acetonitrile) to which are added soluble salts, acids, bases or combinations of these additives. The polar ionic mode is useful for ionizable compounds, where a good starting mobile phase is 0.1% TEA/ 0.1 % acetic acid in methanol. In the case of LC-MS, use volatile ammonium salts, like ammonium formate or ammonium trifluoroacetate. When working with polar ionic mode, method development and optimization involves adjusting the ratio of acid (typically acetic acid) to base (typically TEA) to adjust selectivity and retention. The ratio depends on the pKa of the enantiomers and other factors. For basic analytes, use higher proportion of acid, while for acid analytes, use higher proportion of base. The acid-base ratio can be from 4:1 to 1:4. A 1:1 ratio is used for screening purposes. If the retention is still too short, using a low concentration of TEAA (0.1%) or replacing the methanol with up to 50% acetonitrile will reduce the mobile phase strength. However, if the compound is neutral and not very polar, there will be little or no retention under polar ionic conditions and a different mobile phase system, like normal phase, should be tried.
When is it advisable to add water to mobile phases in polar ionic mode (PIM)?
The ionic functional groups present in CHIROBIOTIC phases means you can use polar additives (salts, water) to improve method development, method reproducibility and column performance. When developing a method for ionizable analytes (acids, bases, zwitterions) on Astec CHIROBIOTIC columns, the polar ionic mode (PIM) is a good starting point. The polar ionic mode mobile phase is methanol containing 0.1% w/v ammonium formate. Vary the NH4OH:formic acid ratio, or try other ammonium salts (e.g. trifluoroacetate or acetate) for optimization. In LC-MS applications, a small amount of water (3-5%) can be added to the mobile phase to improve sensitivity and reproducibility of the method. The addition of water helps to improve the solubility of salts in the methanol.
Chiral GC
What are cyclodextrins?
Cyclodextrins are produced by partial degradation of starch, followed by the enzymatic coupling of glucose units into crystalline, homogeneous toroidal structures of different molecular size. The D(+)-glucose residues are bonded to each other through α-(1,4)glycosidic linkages. The chair configuration of glucose residues makes the toroid “bucket” narrower at one end. Three highly-characterized cyclodextrins are alpha (α), beta (ß), and gamma (γ) cyclodextrin, which contain six, seven, and eight glucose units, respectively. Because each glucose residue has five chiral centers, cyclodextrins are themselves chiral structures. For example, ß-cyclodextrin has 35 chiral centers.
What differentiates your chiral GC line from the competition?
Completeness and scope. We offer the conventional permethylated phases, but we also have polar phases (e.g. Supelco DEX 225 and several CHIRALDEX phases). Altogher, we offer 24 distinct chemistries, in many column dimensions.
What chiral GC columns should I have in my screening protocol?
Based on our experience with leveraging the selectivity differences among the CHIRALDEX and Supelco DEX lines, we recommend the following columns to be included in a comprehensive GC screening protocol:
1. Supelco ß-DEX 110 (Permethyl, 2,3,6-tri-O-methyl)
The ß-DEX 110 is a permethylated phase. It is especially effective for saturated analytes with minimal functionality, saturated cyclics, and saturated bicyclics.
2. Astec CHIRALDEX G-TA (Trifluoroacetyl, 2,6-di-O-pentyl-3-trifluoroacetyl)
The G-TA separates the greatest number of enantiomers, often with high enantioselectivity.
3. Astec CHIRALDEX B-DA (Dialkyl, 2,6-di-O-pentyl-3-methoxy)
The B-DA is best suited for larger multi-ring structures. Often shows enantioreversal of separations done one the 110 (permethylated) phase.
4. Astec CHIRALDEX B-DM (Dimethyl, 2,3-di-O-methyl-6-t-butyl silyl)
The B-DM separates the widest variety of different structural types.
5. Astec CHIRALDEX G-PN (Propionyl, 2,6-di-O-pentyl-3-propionyl)
The G-PN functions like the G-TA but shows higher selectivity toward certain amines (amphetamine, methamphetamine), lactones and epoxides.
6. Astec CHIRALDEX B-PH (Hydroxypropyl, (S)-2-hydroxy propyl methyl ether)
The G-PH is especially effective for saturated analytes with minimal functionality, saturated cyclics, and saturated bicyclics. This phase often shows a reversal of elution order (enantioreversal) compared to the B-DA phase.
Derivatization in chiral GC: Why is it necessary, and what types of derivatives do I choose for what analytes?
Derivatives are needed in GC when the underivatized compounds are thermally instable or not volatile enough to be carried by the gas flow. Some compounds require derivatization for good peak shape. Trifluoroacetic anhydride is used for alcohols and amines, also BSA and BSTFA for those functional groups as well. For acids, methanolic HCl seems to be the way to go and ethylchloroformate may also be used. It is still worth trying the underivatized sample on the columns first just to see if it may work and to also have a comparison.
How do I choose between Supelco Dex and Astec methylated phases?
All of our chiral GC columns have different selectivity, there is no direct overlap. Our permethylated (all available hydroxyl groups methylated) phases are Supelco α-DEX, ß-DEX, and ß-DEX 110 and 120, and CHIRALDEX B-PM. We also have dimethylated phases (Supelco α-DEX, ß-DEX, and γ-DEX 325) and CHIRALDEX B-DM. The Supelco DEX phases are less expensive, if price is a consideration.
There are many 'permethylated' cyclodextrin phases on the market for chiral GC. What are the differences between them?
Permethylation simply means that all available -OH grioups on the CD molecule have been methylated. Beyond that, the phases can differ in the concentration of the derivatized CD in the carrier (if a carrier is used at all), the type of carrier (polysiloxane), the thickness of the layer, the diameter of the capillary tubing, the deactivation method of the capillary wall. All chiral GC phases differ in selectivity, even if they have the same type of phase chemistry.
How do I select a chiral GC column based on compound functional groups?
Astec CHIRALDEX and Supelco DEX lines comprise the widest range of derivatized cyclodextrin (CD) phases for chiral GC separations available today. The 26 different phases cover three different cyclodextrins and nine different derivatives. The phases are grouped into three general categories based on the derivative and types of interactions they are proposed to undergo with analytes. For a general guideline on the choice of chiral GC phase based on the analyte functional group, please consult our Cyclodextrin Capillary GC Column chart (below). Please consult our technical services group if you need any help in deciding which GC CSP is best for your separation.
続きを確認するには、ログインするか、新規登録が必要です。
アカウントをお持ちではありませんか?
