Molecular Detection for Rapid Food Pathogen Testing
Assurance® GDS employs an advanced Polymerase Chain Reaction or PCR-based pathogen screening method to test various pathogens in food and environmental samples. Assurance® GDS is an automated nucleic acid amplification system designed to provide the most accurate and reliable results – validated across a wide range of matrices. It is specifically developed to meet the daily rigors & requirements of food testing laboratories.
Assurance® GDS system for food pathogen detection
The Assurance® GDS genetic detection system combines the latest advancements in molecular detection technology and food microbiology to provide faster results with the increased accuracy required to meet today’s food and environmental testing challenges.
The Assurance® GDS system comprises three simple steps:
- Sample enrichment
- Sample preparation assays utilizing our innovative GDS PickPen® immunomagnetic separation (IMS) device
- PCR analysis with the GDS Rotor-Gene® thermal cycler which includes an automated interpretation of the results
Workflow
Molecular Detection System for Rapid Food Pathogen Testing
Learn how the many advancements in molecular detection technology can improve your lab’s efficiency and overall performance.
- Assurance® GDS utilizes proprietary magnetic particles to capture the targeted living organism from the enriched sample, ensuring valid (robust) PCR reactions for all sample matrices
- The innovative Assurance® GDS PickPen® concentration device quickly & easily collects and transfers the concentrated target pathogens—8 samples at a time
- The patented probes and primers utilize highly conserved target gene sequences
- Multiplex platform allows for the simultaneous detection of multiple targets within each amplification tube
- Patented rotary formats allow for fast cycling
PickPen® IMS technology improves assay sensitivity and detection by concentrating only living targeted bacteria from enrichment broth independently with matrix variability. Results are obtained on the same day or next day, instead of following a multi-day full cultural test and confirmation, improving the time to result according to user’s requirements (e.g., saving time for perishable samples).

Confidently test for key foodborne pathogens, including Salmonella, Listeria spp., Listeria monocytogenes, Cronobacter, E. coli O157:H7 (EHEC), and Shiga toxin-producing E. coli (STEC) with our advanced genetic detection system.
We offer multiple formats, including options that can be fully automated using the Assurance® GDS PickPen® PipetMax® (PPMX) system.
ASSURANCE® GDS System for Fast and Accurate Pathogen Detection
Food pathogen testing by the Assurance® GDS system brings advances in speed, simplicity, specificity and sensitivity.
Watch the step-by-step workflow to better understand the flexibility of the system
Learn how pathogen detection is automated with our GDS PickPen® PipetMax® instrument
Simplicity
From start to finish, the Assurance® GDS system is designed to be simple, flexible and easy to run.
- Easy sample prep with no centrifugation or heating blocks
- Concentration of living cells, removal of background flora and/or inhibitors
- Robust PCR amplification, independent of frequently ambiguous melt curve analysis used after amplification to determine results
- Get faster results (as little as 8 hours) with mEHEC® media – a fast proprietary enrichment, that has been harmonized for use with Salmonella, EHEC, and STEC assays
Speed
Assurance® GDS Rotor-Gene® instrument is an innovative centrifugal air-exchange thermal cycler that is faster than many conventional cyclers.

- The cycler’s constant rotary motion overcomes the heat equilibrium issues inherent in conventional Peltier block systems, lowering dwell time to generate faster results
- Save up to 2 hours of amplification time per run
Specificity
The system’s patented primers and probes ensure greater accuracy. Each amplification tube contains a set of highly specific patented primers, probes and a discrete internal control, which ensures the highest level of specificity.
- Minimizes the risk for cross-reactivity with non-target organisms
- Fewer indeterminate or false positive reactions
- Three levels of specificity give reliable pathogen identification
- Provides greater confidence in results
Sensitivity
Assurance® GDS system delivers a large amount of high-quality DNA for analysis, ensuring a higher degree of sensitivity.
- The innovative patent-pending sample preparation procedure utilizes proprietary magnetic particles to capture and transfer the targeted living bacteria cells from the enriched sample to the amplification tube
- Concentrated sample creates abundant DNA, available for analysis allowing for a shorter enrichment
Assurance® GDS Upgrades: Adaptor Box, RGQ Software and Shipping
The new Assurance® GDS Adaptor Box for the Assurance® GDS Rotor-Gene Q (RGQ) is an optional accessory designed to enhance user interactions, connectivity, and scalability. One of the key features of the adaptor boxes is the ability to connect up to five Assurance® GDS RGQs to a single laptop, saving space and increasing overall system throughput. To accommodate the new accessory, we have released a new Assurance® GDS software version, v2.3.103.23. While it will function similarly to the previous version (one Assurance® GDS RGQ per laptop), there will be interface changes when the Assurance® GDS Adaptor Box is connected. Additionally, our technical services teams are available to assist with the Assurance® GDS Adaptor Box setup.
All new laptops will come pre-installed with this software version, available in regional languages (French, Spanish, and Simplified Chinese), regardless of Assurance® GDS Adaptor Box usage. This software will also include the latest rotor templates with modified logic for the MPX and EHEC ID assays, eliminating the need to upload the 36-rotor template separately as required in version 22.
Advantages:
- Improved Assurance® GDS RGQ connectivity and scalability
- Prevents lost runs
- Sample throughput of up to 180 samples (connects five Assurance® GDS RGQs to one laptop)
- Allows staging of runs while Assurance® GDS RGQ is operating
- Optional Wi-Fi connection
- Assurance® GDS Software available in local languages (Spanish, French, simp. Chinese)
The Assurance® GDS RGQ software version can be downloaded here.
New Shipping Information
Recently, we conducted a shipping shelf-life study for Assurance® GDS assays to simulate and evaluate the effect of short-term temperature exposure during shipping, handling, and storage of Assurance® GDS Kits. According to the new stability study, Assurance® GDS assay efficacy did not negatively impact the stability or the efficacy of the product for up to three weeks at 25 °C and for up to one week at 45 °C. However, the optimal Assurance® GDS assay storage temperature remains under refrigeration, 2 to 8 °C.
MicroVal and AOAC extension for Assurance® GDS Cronobacter II confirmation
An extension for a new rapid confirmation method for Assurance® GDS for Cronobacter Tq II was performed in infant nutrition products, milk powder, and environmental samples, comparing it to the ISO reference method. This study followed the MicroVal interpretation guidelines for ISO 16140-Part 6 (2019) as an extension of a previously validated qualitative method. The findings were obtained using both direct streak and IMS methodologies for isolating and confirming Cronobacter. These two new methods offer rapid isolation and confirmation, ensuring selectivity and specificity in line with the Assurance® GDS for Cronobacter Tq II detection method.
For more information, please read the application note.

Explore our range of Assurance® GDS kits for testing pathogens in food and environmental samples. Click on the catalog number to learn more.
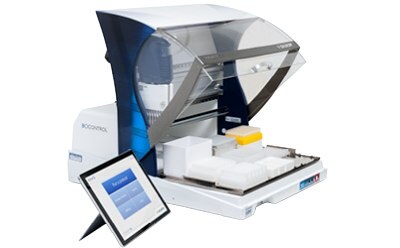
Automation of sample preparation for the Assurance® GDS method at a size and price accessible to any lab.
See how the GDS PickPen® PipetMax® instrument has been programmed to automate GDS PickPen® IMS procedures and transfers to amplification tubes.
The GDS PickPen® PipetMax® instrument easily automates routine sample preparation and pathogen detection, saving time and allowing you to focus on other critical laboratory tasks via several key features:
- Flexible modular design accommodates existing lab layout and workflows
- Efficient protocols for small and large runs to maximize productivity
- Reduces PCR sample preparation labor by up to 50% and removes user variation by standardizing results
- Small footprint saves valuable lab bench space and manages cost
続きを確認するには、ログインするか、新規登録が必要です。
アカウントをお持ちではありませんか?