B0125
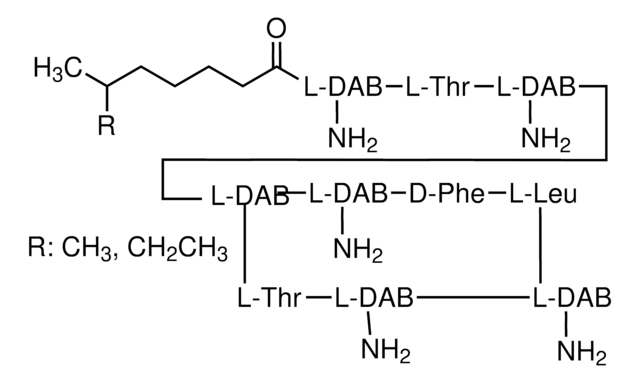
Bacitracin
from Bacillus licheniformis, ≥65 IU/mg
Synonym(s):
Altracin, Bacitracin A
About This Item
Recommended Products
biological source
Bacillus licheniformis
Quality Level
form
powder
specific activity
≥65 IU/mg
antibiotic activity spectrum
Gram-positive bacteria
Mode of action
cell wall synthesis | interferes
storage temp.
2-8°C
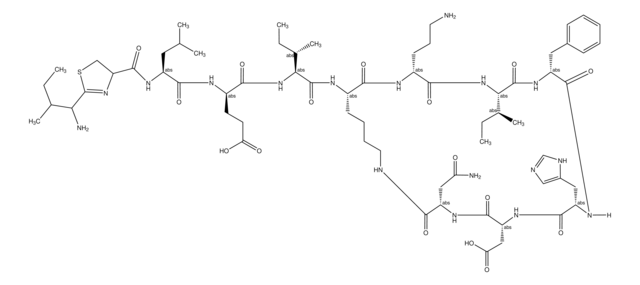
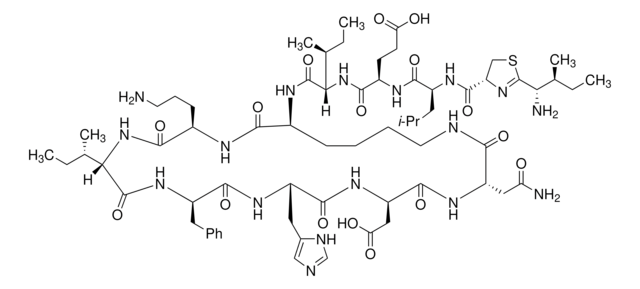
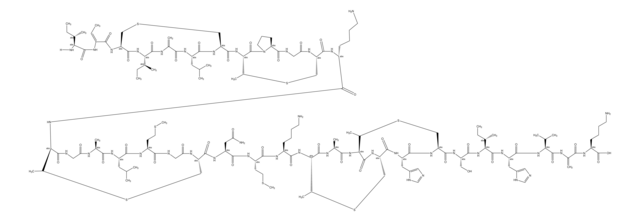
SMILES string
[H]N1[C@@H](Cc2cnc[nH]2)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)NCCCC[C@H](NC(=O)[C@@H](NC(=O)[C@@H](CCC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)C3CSC(=N3)C(N)C(C)CC)[C@@H](C)CC)C(=O)N[C@H](CCCN)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@H](Cc4ccccc4)C1=O
InChI
1S/C66H103N17O16S/c1-9-35(6)52(69)66-81-48(32-100-66)63(97)76-43(26-34(4)5)59(93)74-42(22-23-50(85)86)58(92)83-53(36(7)10-2)64(98)75-40-20-15-16-25-71-55(89)46(29-49(68)84)78-62(96)47(30-51(87)88)79-61(95)45(28-39-31-70-33-72-39)77-60(94)44(27-38-18-13-12-14-19-38)80-65(99)54(37(8)11-3)82-57(91)41(21-17-24-67)73-56(40)90/h12-14,18-19,31,33-37,40-48,52-54H,9-11,15-17,20-30,32,67,69H2,1-8H3,(H2,68,84)(H,70,72)(H,71,89)(H,73,90)(H,74,93)(H,75,98)(H,76,97)(H,77,94)(H,78,96)(H,79,95)(H,80,99)(H,82,91)(H,83,92)(H,85,86)(H,87,88)/t35?,36-,37-,40-,41+,42+,43-,44+,45-,46-,47+,48?,52?,53-,54-/m0/s1
InChI key
CLKOFPXJLQSYAH-RNHDWVCBSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Biochem/physiol Actions
Antimicrobial spectrum: Gram-positive bacteria.
Mode of Action: Inhibits bacterial cell wall synthesis by inhibiting dephosphorylation of lipid pyrophosphate.
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
β-lactam antibacterials inhibit transpeptidase enzymes, preventing peptidoglycan assembly in both Gram-positive and Gram-negative bacteria.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service