157716
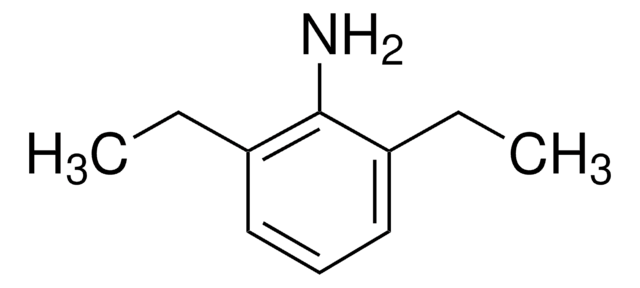
2,6-Diisopropylaniline
technical grade, 90%
동의어(들):
2,6-Bis(1-methylethyl)benzenamine, 2,6-Bis(propan-2-yl)aniline, 2,6-Diisopropylphenylamine
로그인조직 및 계약 가격 보기
크기 선택
모든 사진(3)
크기 선택
보기 변경
About This Item
Linear Formula:
[(CH3)2CH]2C6H3NH2
CAS Number:
Molecular Weight:
177.29
Beilstein:
2208763
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Grade
technical grade
Quality Level
vapor pressure
<0.01 mmHg ( 20 °C)
분석
90%
양식
liquid
refractive index
n20/D 1.532 (lit.)
bp
257 °C (lit.)
mp
−45 °C (lit.)
density
0.94 g/mL at 25 °C (lit.)
SMILES string
CC(C)c1cccc(C(C)C)c1N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
2,6-Diisopropylaniline on condensation with triacetylmethane in toluene in the presence of p-toluenesulfonic acid provides 3-[1-(2,6-diisopropylphenylamino)ethylidene]pentane-2,4-dione.
애플리케이션
2,6-Diisopropylaniline was used in the preparation of multitopic Schiff-base ligand precursors. It was also used in the preparation of NSN-donor proligand, 4,5-bis(2,6-diisopropylanilino)-2,7-di-tert-butyl-9,9-dimethylthioxanthene.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Chronic 3 - Eye Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
개인 보호 장비
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
이미 열람한 고객
Yilin Sun et al.
Dalton transactions (Cambridge, England : 2003), 41(32), 9682-9688 (2012-07-12)
Four multitopic Schiff-base ligand precursors were synthesized via condensation of 4,4'-diol-3,3'-diformyl-1,1'-diphenyl or 1,3,5-tris(4-hydroxy-5-formylphenyl)benzene with 2,6-diisopropylaniline or 2,6-dimethylaniline. Amine elimination reactions of Ln[N(SiMe(3))(2)](3) (Ln = La, Nd, Sm or Y) with these multitopic ligand precursors gave ten heterogeneous rare-earth metal catalysts.
Balamurugan Vidjayacoumar et al.
Dalton transactions (Cambridge, England : 2003), 41(26), 8175-8189 (2012-05-09)
A rigid NSN-donor proligand, 4,5-bis(2,6-diisopropylanilino)-2,7-di-tert-butyl-9,9-dimethylthioxanthene (H(2)[TXA(2)], 1) was prepared by palladium-catalyzed coupling of 2,6-diisopropylaniline with 4,5-dibromo-2,7-di-tert-butyl-9,9-dimethylthioxanthene. Deprotonation of 1 using (n)BuLi provided Li(2)(DME)(2)[TXA(2)] (2), and subsequent reaction with UCl(4) afforded [Li(DME)(3)][(TXA(2))UCl(3)] (4). The analogous NON-donor ligated complex [(XA(2))UCl(3)K(DME)(3)] [3; XA(2)
Remote activation of nickel catalysts for ethylene oligomerization.
Yaofeng Chen et al.
Angewandte Chemie (International ed. in English), 44(7), 1108-1112 (2005-01-13)
D A Razborov et al.
Dalton transactions (Cambridge, England : 2003), 44(47), 20532-20541 (2015-11-10)
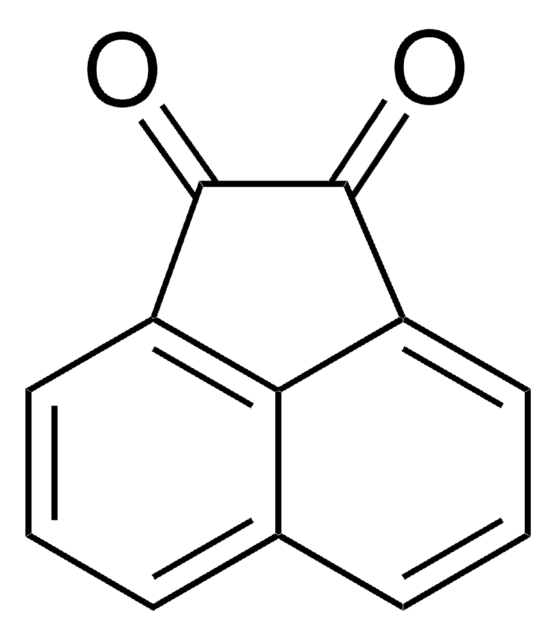
In the presence of formic acid, acenaphthenequinone (AQ) reacts with one molar equivalent of 2,6-diisopropylaniline in toluene to give monoiminoacenaphtheneone (3, dpp-mian) in good yield. Reduction of compound 3 with an excess of magnesium in thf results in green crystalline
Luka A Wright et al.
Dalton transactions (Cambridge, England : 2003), 44(16), 7230-7241 (2015-03-20)
The 2-(2′-aniline)-6-imine-pyridines, 2-(C6H4-2′-NH2)-6-(CMe=NAr)C5H3N (Ar = 4-i-PrC6H4 (HL1a), 2,6-i-Pr2C6H3 (HL1b)), have been synthesised via sequential Stille cross-coupling, deprotection and condensation steps from 6-tributylstannyl-2-(2-methyl-1,3-dioxolan-2-yl)pyridine and 2-bromonitrobenzene. The palladium(II) acetate N,N,N-pincer complexes, [{2-(C6H4-2′-NH)-6-(CMe=NAr)C5H3N}Pd(OAc)] (Ar = 4-i-PrC6H4 (1a), 2,6-i-Pr2C6H3 (1b)), can be prepared by
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.