20021
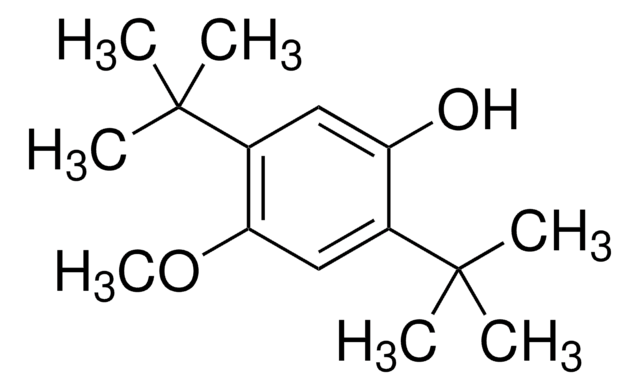
3-tert-Butyl-4-hydroxyanisole
≥98% (sum of isomers, GC), ≤10% 2-BHA basis (GC)
동의어(들):
2-tert-Butyl-4-methoxyphenol, 3-BHA, BHA
About This Item
추천 제품
분석
≥98% (sum of isomers, GC)
양식
solid
구성
2-BHA, ≤10% GC
3-BHA, ≥90% GC
불순물
≤1% 4-hydroxyanisole
무기 잔류물
≤0.05%
mp
48-63 °C
solubility
ethanol: soluble 1 g/10 mL, clear, colorless to faint yellow or tan
SMILES string
COc1ccc(O)c(c1)C(C)(C)C
InChI
1S/C11H16O2/c1-11(2,3)9-7-8(13-4)5-6-10(9)12/h5-7,12H,1-4H3
InChI key
MRBKEAMVRSLQPH-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
- Regulation of Smad signaling in mesenchymal stem cells: 3-tert-Butyl-4-hydroxyanisole disrupts the differentiation of C3H10T1/2 mesenchymal stem cells into brown adipocytes by modulating Smad signaling pathways, with potential implications for obesity and metabolic syndrome research (Wang et al., 2023).
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Chronic 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.