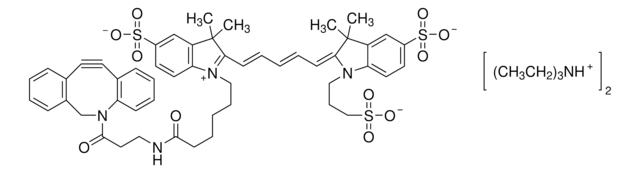
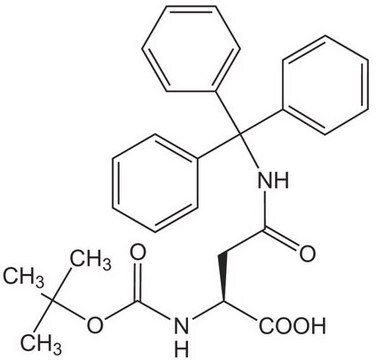
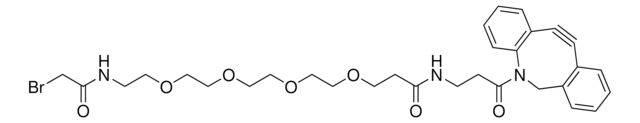
추천 제품
Quality Level
분석
≥94.5 %
양식
solid
반응 적합성
reaction type: click chemistry
reagent type: linker
mp
86-96 °C
작용기
amine
저장 온도
−20°C
SMILES string
NCCC(N1CC2=C(C=CC=C2)C#CC3=C1C=CC=C3)=O
InChI
1S/C18H16N2O/c19-12-11-18(21)20-13-16-7-2-1-5-14(16)9-10-15-6-3-4-8-17(15)20/h1-8H,11-13,19H2
InChI key
OCCYFTDHSHTFER-UHFFFAOYSA-N
일반 설명
Dibenzocyclooctyne-amine (DBCO-NH2), is a heterobifunctional linker containing a DBCO moiety commonly used for the site-specific functionalization of nanobodies, enabling the addition of reactive DBCO groups for subsequent click chemistry reactions.
애플리케이션
Dibenzocyclooctyne-amine can be used as a :
- Linker to link azide-containing functional groups. It facilitates the formation of linkages through Strain-promoted azide-alkyne cycloaddition reactions (SPAAC)
- Reagent in the synthesis of N-heterocyclic carbene metal thiolates. It functionalizes the metal complexes and increases their reactivity in the strain-promoted alkyne–azide cycloaddition (SPAAC) reactions
- Amine functionalized cyclooctyne derivative. Cyclooctynes are useful in strain-promoted copper-free azide-alkyne cycloaddition reactions. This dibenzocyclooctyne will react with azide functionalized compounds or biomolecules without the need for a Cu(I) catalyst to result in a stable triazole linkage.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Catalyst-free site-specific surface modifications of nanocrystalline diamond films via microchannel cantilever spotting
Davydova M, et al.
Royal Society of Chemistry Advances, 6(63), 57820-57827 (2016)
An azide functionalized oligothiophene ligand?A versatile tool for multimodal detection of disease associated protein aggregates.
Johansson LB, et al.
Biosensors And Bioelectronics, 63, 204-211 (2015)
Picomolar SARS-CoV-2 neutralization using multi-arm PEG nanobody constructs
A Moliner-Morro, et al.
Biomolecules, 10, 1661-1661 (2020)
Covalently linking CuInS 2 quantum dots with a Re catalyst by click reaction for photocatalytic CO 2 reduction
J Huang, et al.
Dalton Transactions, 47, 10775-10783 (2018)
NHC ligated group 11 metal-arylthiolates containing an azide functionality amenable to ?click? reaction chemistry
V Somasundaram, et al.
Inorganic Chemistry, 57, 11184-11192 (2018)
문서
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Explore the principles and applications of click chemistry in drug discovery, highlighting efficient reactions that streamline the synthesis of bioactive compounds.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
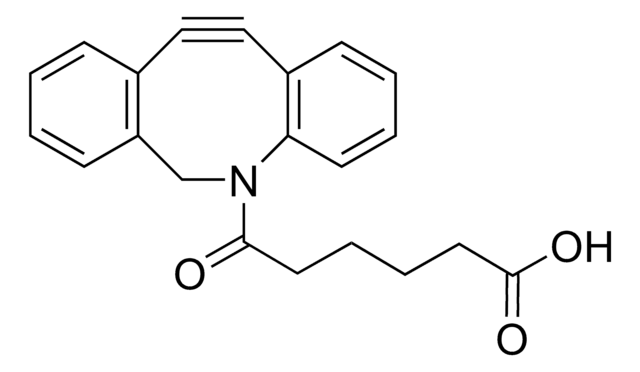
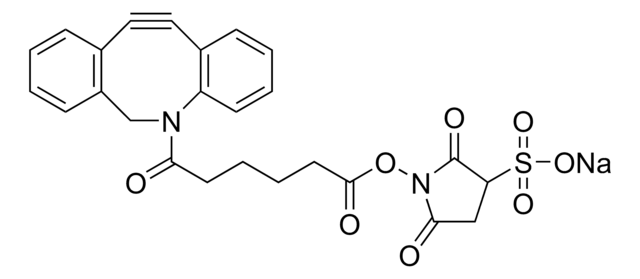
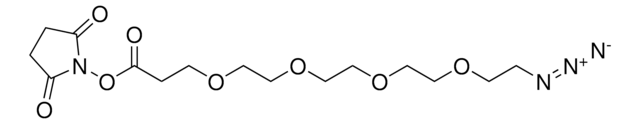
![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)
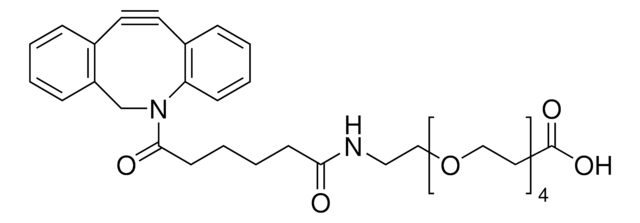
![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethanol for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/171/632/0556139a-2db5-4678-a6ec-a26a693fd574/640/0556139a-2db5-4678-a6ec-a26a693fd574.png)
![N-[(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyloxycarbonyl]-1,8-diamino-3,6-dioxaoctane for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/294/853/c5e47d84-5aee-4797-aa24-604f291171cc/640/c5e47d84-5aee-4797-aa24-604f291171cc.png)