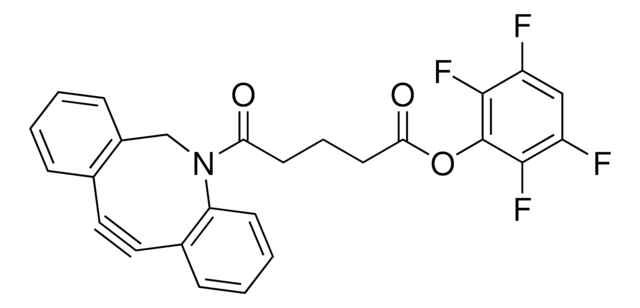
761516
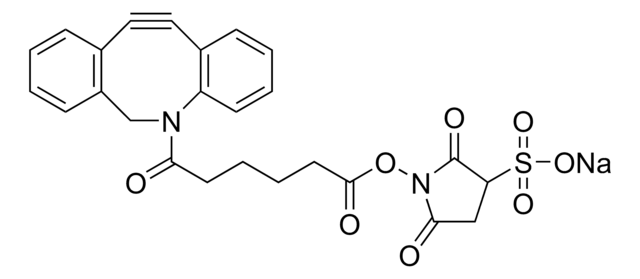
Dibenzocyclooctyne-acid
95%, storage temp.:-20°C
Synonym(s):
DBCO-Acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C21H19NO3
Molecular Weight:
333.38
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
95%
form
solid
reaction suitability
reaction type: click chemistry
reagent type: linker
mp
118-125 °C
functional group
carboxylic acid
storage temp.
−20°C
SMILES string
O=C(CCCCC(O)=O)N1CC2=C(C=CC=C2)C#CC3=C1C=CC=C3
InChI
1S/C21H19NO3/c23-20(11-5-6-12-21(24)25)22-15-18-9-2-1-7-16(18)13-14-17-8-3-4-10-19(17)22/h1-4,7-10H,5-6,11-12,15H2,(H,24,25)
InChI key
NIRLBCOFKPVQLM-UHFFFAOYSA-N
General description
Acid functionalized cyclooctyne derivative. Cyclooctynes are useful in strain-promoted copper-free azide-alkyne click chemistry reactions. This azadibenzocyclooctyne will react with azide functionalized compounds or biomolecules without the need for a Cu(I) catalyst to result in a stable triazole linkage.
Application
Dibenzocyclooctyne-acid may be used for the surface modification of eight-arm poly(ethylene glycol), to make it susceptible to strain promoted alkyne-azide cycloaddition (SPAAC) with PEG-bis-azide leading to the formation of hydrogels. These hydrogels are useful for protein immobilization.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ratiometric Fluorescence Azide?Alkyne Cycloaddition for Live Mammalian Cell Imaging.
Fu H, et al.
Analytical Chemistry, 87(22), 11332-11336 (2015)
Chen Guo et al.
Acta biomaterialia, 56, 80-90 (2017-04-10)
Hydrogels are facile architectures for the controlled presentation of proteins with far-reaching applications, from fundamental biological studies in three-dimensional culture to new regenerative medicine and therapeutic delivery strategies. Here, we demonstrate a versatile approach for spatially-defined presentation of engineered proteins
Flexible synthesis of cationic peptide?porphyrin derivatives for light-triggered drug delivery and photodynamic therapy.
Dondi R, et al.
Organic & Biomolecular Chemistry, 14(48), 11488-11501 (2016)
Seyed Mohammad Mahdi Dadfar et al.
Small (Weinheim an der Bergstrasse, Germany), 14(21), e1800131-e1800131 (2018-04-24)
Different types of click chemistry reactions are proposed and used for the functionalization of surfaces and materials, and covalent attachment of organic molecules. In the present work, two different catalyst-free click approaches, namely azide-alkyne and thiol-alkyne click chemistry are studied
Near-infrared light-triggered thermochemotherapy of cancer using a polymer?gold nanorod conjugate.
Ko H, et al.
Nanotechnology, 27(17), 175102-175102 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service