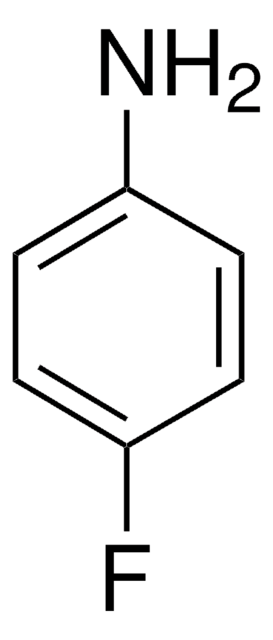
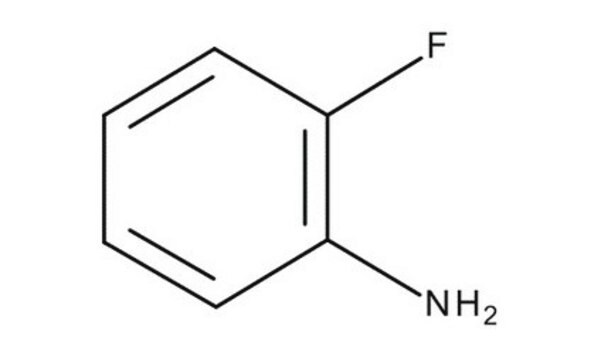
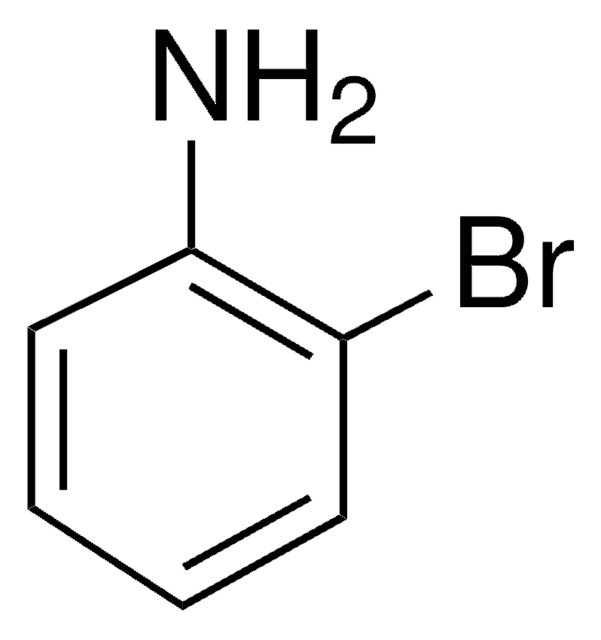
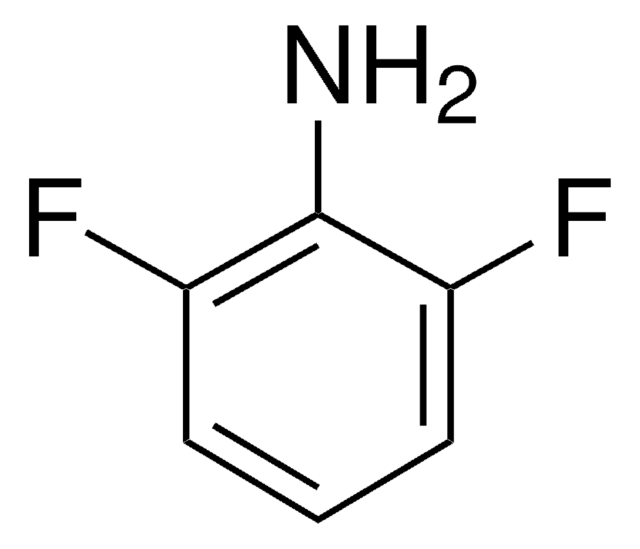
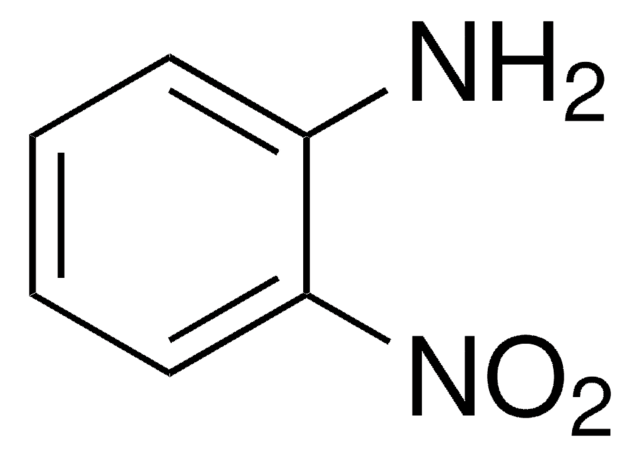
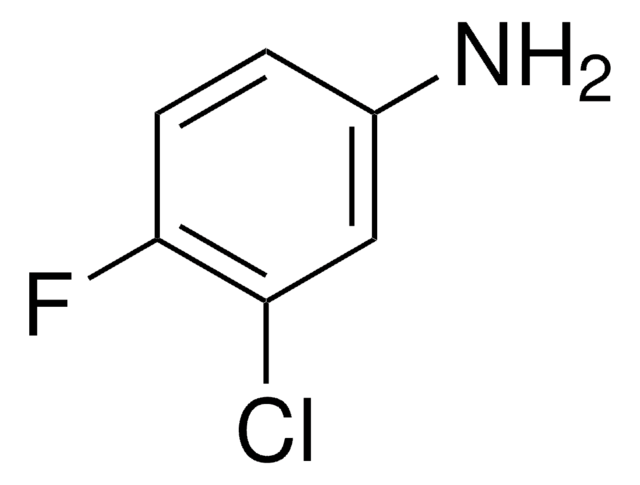
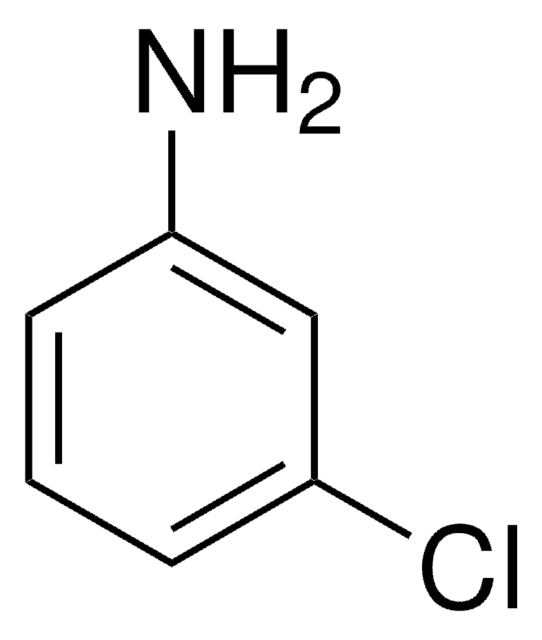
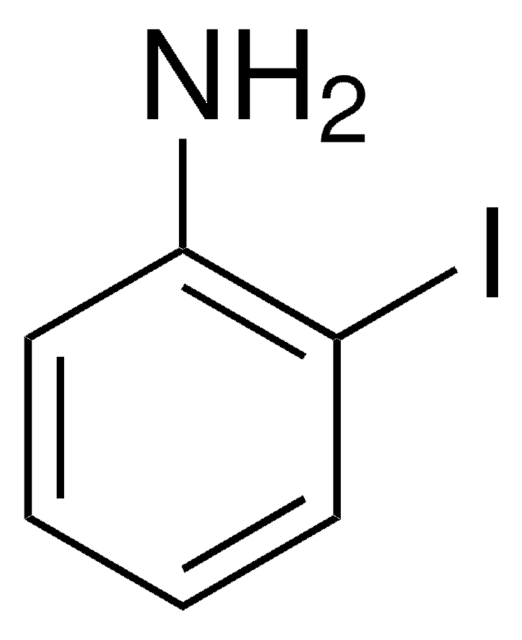
추천 제품
분석
≥99%
refractive index
n20/D 1.544 (lit.)
bp
182-183 °C (lit.)
mp
−29 °C (lit.)
density
1.151 g/mL at 25 °C (lit.)
SMILES string
Nc1ccccc1F
InChI
1S/C6H6FN/c7-5-3-1-2-4-6(5)8/h1-4H,8H2
InChI key
FTZQXOJYPFINKJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
생화학적/생리학적 작용
The metabolism and excretion of the xenobiotic compound 2-fluoroaniline is important due to human exposure in manufacturing. It is found to be very efficiently metabolized, primarily by 4-hydroxylation with subsequent sulfate or glucuronide formation. N-Acetylation is also observed. At least 80% of the dose is excreted in the urine within 24 hr. 2-Fluoroaniline exerts its nephrotoxic effect through 4-hyroxylation and subsequent p-benzoquinonimine formation.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1C - STOT RE 2
표적 기관
Blood,hematopoietic system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point (°F)
140.0 °F - closed cup
Flash Point (°C)
60 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Suria Jahan et al.
Transfusion, 60(4), 769-778 (2020-03-19)
Platelet engraftment following cord blood (CB) transplantation remains a significant hurdle to this day. The uncontrolled growth of ice, a process referred to as ice recrystallization, is one of several mechanisms that lead to cell loss and decreased potency during
J Vervoort et al.
NMR in biomedicine, 4(6), 255-261 (1991-12-01)
The present study describes results from an in vivo 19F NMR study on rats exposed to the xenobiotic compound 2-fluoroaniline. Qualitative pharmacokinetics and the biotransformation of 2-fluoroaniline were studied after exposure to 50 mg/kg body wt 2-fluoroaniline. Accumulation and elimination
M A Valentovic et al.
Toxicology, 75(2), 121-131 (1992-11-01)
Aniline and its halogenated derivatives are widely used as chemical intermediates. The purpose of this study was to determine the hepatotoxic and nephrotoxic potential of the 2-haloanilines. Male Fischer 344 rats (n > or = 4) were injected (i.p.) with
A L Sharma et al.
Applied biochemistry and biotechnology, 96(1-3), 155-165 (2002-01-11)
Poly(2-fluoroaniline) was prepared by both chemical and electrochemical polymerization in acidic medium. Characterization of poly(2-fluoroaniline) was accomplished experimentally using ultraviolet-visible, Fourier transform infrared, differential scanning calorimetry, thermal gravimetric analysis, and X-ray diffraction techniques, respectively. Scanning electron microscopy studies revealed globular
M Arivazhagan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 96, 668-676 (2012-08-14)
The Fourier transform infrared (FT-IR) and Fourier transform Raman (FT-Raman) spectra of 4-chloro-2-fluoroaniline (CFA) have been recorded and analyzed. The equilibrium geometry, bonding features and harmonic vibrational frequencies have been investigated with the help of ab initio and density functional
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.