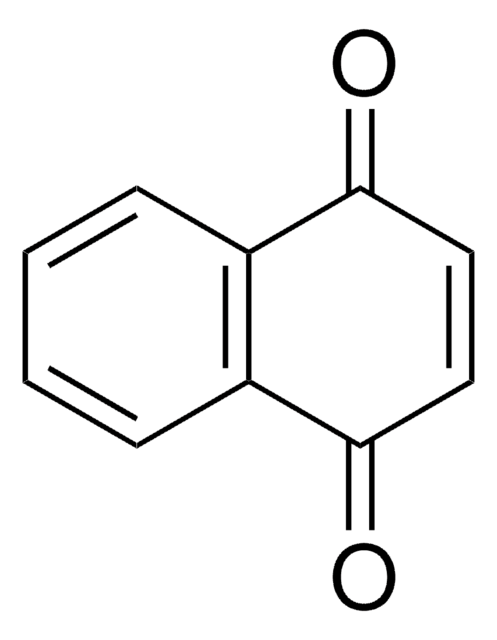
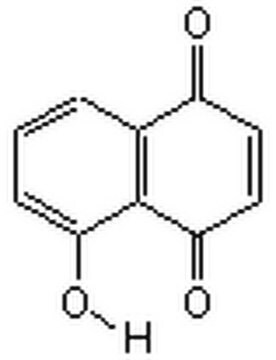
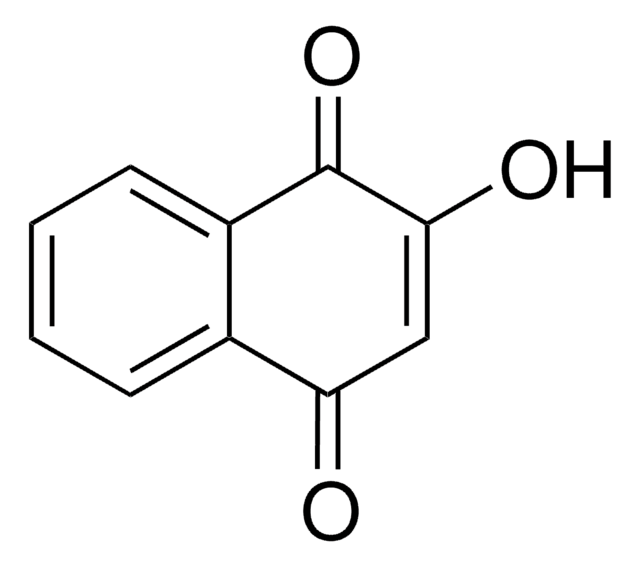
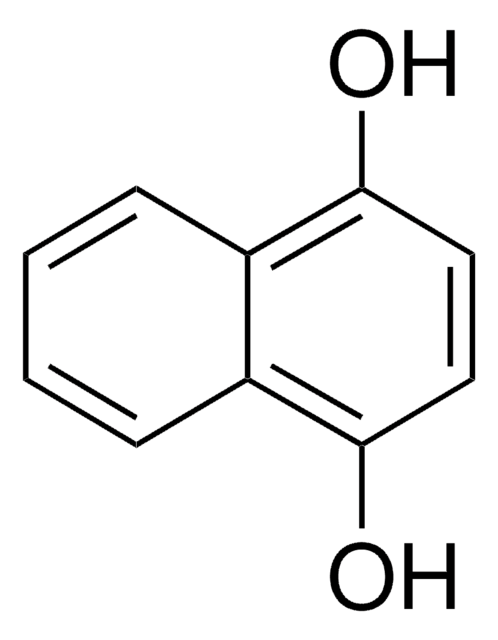
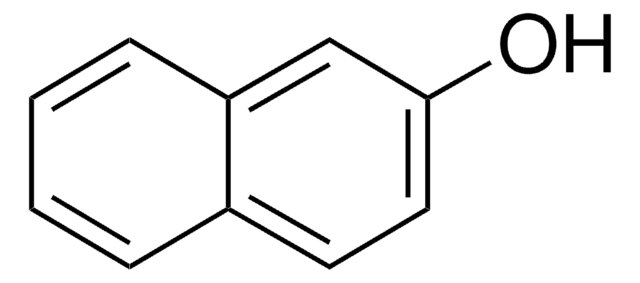
추천 제품
grade
purum
Quality Level
분석
≥96.5% (HPLC)
양식
powder
mp
119-122 °C (lit.)
120-124 °C
작용기
ketone
SMILES string
O=C1C=CC(=O)c2ccccc12
InChI
1S/C10H6O2/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6H
InChI key
FRASJONUBLZVQX-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
It can be used to synthesize:[2]
- 3,3-Disubstituted oxindoles via asymmetric Michael addition to oxindole.
- Bioactive isoindolines via asymmetric 1,3-dipolar cycloaddition to azomethine ylides generated in situ from aldehydes and diethyl aminomalonate.
- α,α-Difluoro-β-hydroxy ketone via ‘on water′ catalyst-free Mukaiyama-aldol reaction with difluoroenoxysilane.
- 2-Hydroxy-3-anilino-1,4-naphthoquinone, which shows potent in vivo antimalarial activity.
Additional appilcation include:[2]
- As an arylation reagent for the α-arylation of aldehydes.
- As a starting material in the multi-step synthesis of benz[f]indole-4,9-diones.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1C - Skin Sens. 1 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
285.8 °F
Flash Point (°C)
141 °C
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.