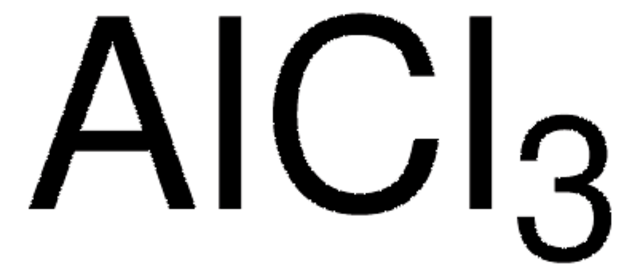추천 제품
일반 설명
Potassium acetate is an osmotic agent that can be prepared by the reaction between acetic acid and potassium hydroxide. It has replaced urea and glycol for deicing process. Its potential as a substitute to calcium chloride for osmotic distillation has been investigated. It intercalates with the hydroxyl groups on the kaolinite surface leading to surface modification. It acts as a catalyst that accelerates the acetylation of wood at low temperature.
애플리케이션
Potassium acetate has been used during granule bound starch synthase (GBSS) activity assay. It may be used as an activator to produce waste tea activated carbon (WTAC).
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
적합한 제품을 찾을 수 없으신가요?
당사의 제품 선택기 도구.을(를) 시도해 보세요.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Modification of kaolinite surfaces through intercalation with potassium acetate, II.
Frost RL, et al.
Journal of Colloid and Interface Science, 214(1), 109-117 (1999)
Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption of Acid Blue 25 dye.
Auta M and Hameed BH.
Chemical Engineering Journal, 171(2), 502-509 (2011)
Potassium acetate-catalyzed acetylation of wood: reaction rates at low temperatures.
Obataya E and Minato K.
Wood Science and Technology, 43(5-6), 405-413 (2009)
Purification of starch granules from Arabidopsis leaves and determination of granule-bound starch synthase activity.
Albi T, et al.
Bio-protocol, 4(23), e1316-e1316 (2013)
M M Shah et al.
Applied biochemistry and biotechnology, 63-65, 423-433 (1997-04-01)
Potassium acetate is currently made by reacting petroleum-based acetic acid with potassium hydroxide. An alternate process, anaerobic fermentation of dextrose with Clostridium thermoaceticum, could be used and could possibly be cheaper. Growth characteristics and productivity of the fermentation were optimized
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.