가격 및 재고 정보를 현재 이용할 수 없음
추천 제품
Quality Level
분석
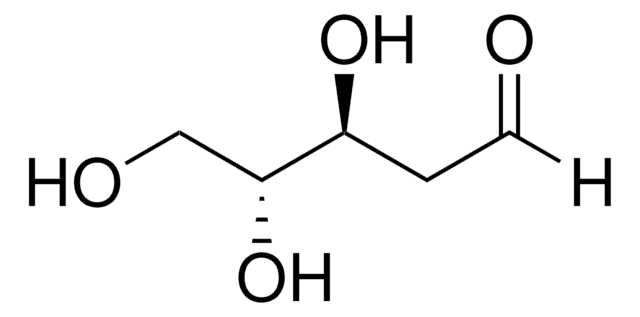
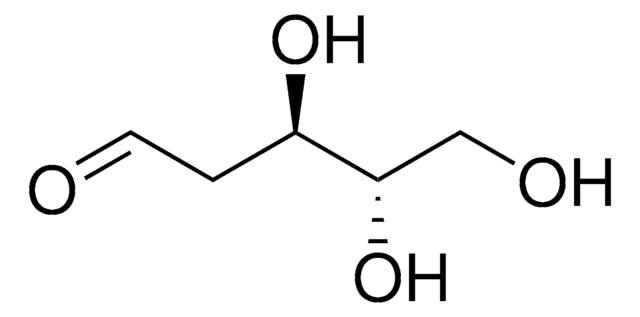
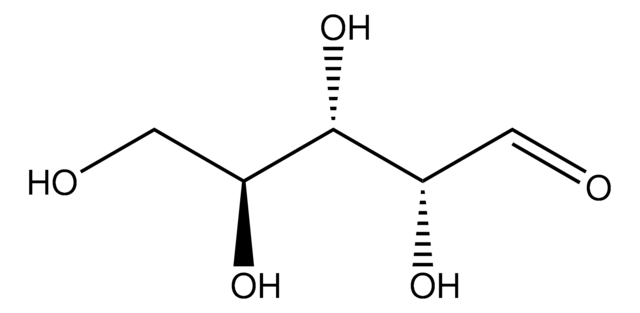
≥99.0% (TLC)
양식
solid
광학 활성
[α]20/D −56±2°, 24 hr, c = 1% in H2O
불순물
<0.5% Sulphated ash
무기 잔류물
≤0.5% (as SO4)
손실
≤1% loss on drying, 20 °C (HV)
색상
white
mp
89-90 °C (lit.)
solubility
water: 50 mg/mL, clear, colorless to faintly yellow
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
2-Deoxy-D-ribose is used to study processes of oxidative stress and glycation in vivo and in vitro. 2-Deoxy-D-ribose, an endothelial-cell chemoattractant and angiogenesis-inducing factor, is used to study processes of tumor angiogenesis and progression mediated at the level of thymidine phosphorylase activity.
기타 정보
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Xican Li
Food chemistry, 141(3), 2083-2088 (2013-07-23)
The deoxyribose degradation assay is widely used to evaluate the hydroxyl (OH) radical-scavenging ability of food or medicines. We compared the hydroxyl radical-scavenging activity of 25 antioxidant samples prepared in ethanol solution with samples prepared after removing the ethanol (residue).
Jean Cadet et al.
Free radical research, 46(4), 367-381 (2012-01-24)
A broad scientific community is involved in investigations aimed at delineating the mechanisms of formation and cellular processing of oxidatively generated damage to nucleic acids. Perhaps as a consequence of this breadth of research expertise, there are nomenclature problems for
Marina Rossi et al.
Physical review letters, 110(10), 107801-107801 (2013-03-26)
Concentrated solutions of ultrashort duplex-forming DNA oligomers may develop various forms of liquid crystal ordering among which is the chiral nematic phase, characterized by a macroscopic helical precession of molecular orientation. The specifics of how chirality propagates from the molecular
N S Brown et al.
The Biochemical journal, 334 ( Pt 1), 1-8 (1998-08-07)
Angiogenesis is the term used to describe the formation of new blood vessels from the existing vasculature. In order to attract new vessels, a tissue must release an endothelial-cell chemoattractant. 2-Deoxy-D-ribose is produced in vivo by the catalytic action of
Guillaume Mata et al.
The Journal of organic chemistry, 77(20), 9006-9017 (2012-09-15)
An efficient method for the N-2-deoxyribosylation of modified nucleobases by 2-deoxythioriboside donors is reported. In the presence of an in situ silylated nucleobase, thioglycosides can be activated with NIS/HOTf to give nucleosides in high yields and with good β-selectivity. By
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.