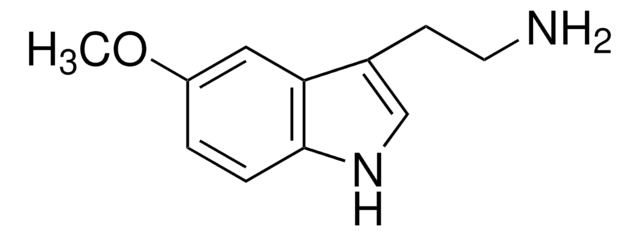
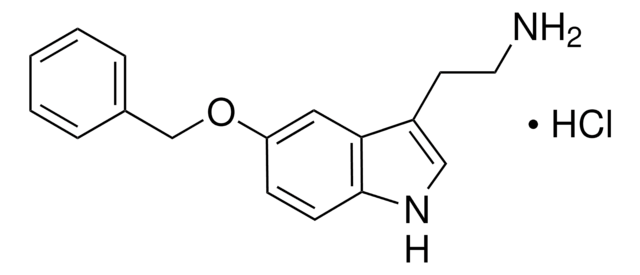
추천 제품
제품명
N-Acetyl-5-hydroxytryptamine, ≥99% (HPLC), powder
Quality Level
분석
≥99% (HPLC)
양식
powder
색상
white to faint yellow
mp
120-122 °C (lit.)
solubility
ethanol: 50 mg/mL
저장 온도
2-8°C
SMILES string
CC(=O)NCCc1c[nH]c2ccc(O)cc12
InChI
1S/C12H14N2O2/c1-8(15)13-5-4-9-7-14-12-3-2-10(16)6-11(9)12/h2-3,6-7,14,16H,4-5H2,1H3,(H,13,15)
InChI key
MVAWJSIDNICKHF-UHFFFAOYSA-N
유전자 정보
human ... MTNR1A(4543) , MTNR1B(4544)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
N-Acetylserotonin (NAS)/normelatonin acts as a precursor of melatonin in the tryptophan metabolic pathway.
애플리케이션
N-Acetyl-5-hydroxytryptamine has been used to treat neuron cells and to analyze its effects on Krüppel-like factor 15 (KLF15) expression.
생화학적/생리학적 작용
N-acetyl-serotonin (NAS/normelatonin) can act as a shelter to neurons due to its protecting ability against oxidative challenges. It can also repress the actions of the transcription factor NF-kappaB. NAS possesses antioxidant and antiaging actions. It has protective action against β-amyloid induced neurotoxicity. It helps to maintain the optimal fluidity of the biological membranes.
Immediate precursor of melatonin. It is formed from serotonin and acetyl-CoA in a reaction catalyzed by serotonin N-acetyl transferase, the rate-limiting enzyme in melatonin biosynthesis. This indoleamine is a weak agonist at melatonin receptors, and has moderate effects on G-protein stimulation and inhibition of cAMP accumulation.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Jimo Borjigin et al.
Molecular and cellular endocrinology, 349(1), 13-19 (2011-07-26)
The pineal gland is a neuroendocrine organ of the brain. Its main task is to synthesize and secrete melatonin, a nocturnal hormone with diverse physiological functions. This review will focus on the central and pineal mechanisms in generation of mammalian
J K Yao et al.
Molecular psychiatry, 15(9), 938-953 (2009-04-30)
Schizophrenia is characterized by complex and dynamically interacting perturbations in multiple neurochemical systems. In the past, evidence for these alterations has been collected piecemeal, limiting our understanding of the interactions among relevant biological systems. Earlier, both hyper- and hyposerotonemia were
Izabela Sadowska-Woda et al.
Toxicology in vitro : an international journal published in association with BIBRA, 24(3), 879-884 (2009-12-08)
beta-Cyfluthrin is one of the most widely used type II pyrethroid in agriculture. The aim of this study was to examine (1) the possibility of beta-cyfluthrin to induce oxidative stress in human erythrocytes in vitro and its effect on catalase
N-Acetylserotonin and 6-hydroxymelatonin against oxidative stress: Implications for the overall protection exerted by melatonin
A?lvarez DR, et al.
The Journal of Physical Chemistry B, 119(27), 8535-8543 (2015)
C S Pang et al.
Biological signals, 4(6), 311-324 (1995-11-01)
We have compared the pharmacological characteristics of 2-[125I]iodomelatonin binding to crude membrane preparations of the lung, spleen, brain and kidney of chicken. Saturation studies indicated significant differences (p < 0.05) in the equilibrium dissociation constant (Kd) and maximum number of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.