Fluorination
Fluorination, or the introduction of fluorine to a compound, has the profound ability to alter its biological properties due to the electronegativity and small size of fluorine. In pharmaceutical research, fluorine is often introduced into the target compound to improve bioavailability and enhance the metabolic stability profile. The field has grown to include numerous strategies for introduction for the fluorine, including several key reactions.
Featured Categories
Browse through our comprehensive range of solvents offered under three portfolio brands for your various applications: Supelco® products for analytical chemistry, Sigma-Aldrich® materials for lab and production, SAFC® products for biopharmaceutical and pharmaceutical development and manufacturing.
Find the basic components needed to drive your research forward in our portfolio of organic building blocks which include Alkanes, Alkenes, Alkynes, Allenes, Arenes, Arsenic Compounds, Baran Functionalized Building Blocks, Baran Hindered Amines, Building Blocks and Chiral Small Molecules from Liverpool ChiroChem, Building Blocks for SuFEx: The Next Click Reaction, Carbenium Salts, and Carbonyl Compounds.
With a vast offering of fluorinated building blocks, such as, trifluoromethyl, difluoromethyl, triflate, and pentafluorosulfanyl substituents for your toolkit, we make it even easier to discover your target compounds.
Choose from our wide array of halogenated substrates to improve the success of your bromination, chlorination, fluorination, haloboration, and iodination research endeavors.

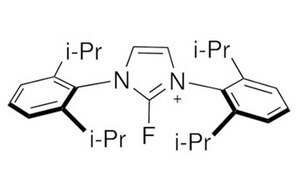
Nucleophilic Fluorination
Nucleophilic fluorination methods employ a fluoride source, such as alkali or ammonium fluoride, for direct displacement of alcohols, additions to aldehydes, ketones and carboxylic acids in highly chemoselective fashions for small molecule synthesis, as well as polyfluorination for materials synthesis.

Electrophilic Fluorination
Electrophilic fluorination involves the combination of a carbon-centered nucleophile with an electrophilic source of fluorine. Traditionally, the source of electrophilic fluorine used was fluorine gas, which is highly toxic and a strong oxidizer. However, research has led to reagents that are milder, safer and highly stable alternatives for electrophilic fluorination. These reagents have shown excellent utility in various applications, ranging from electrophilic aromatic substitution to formation of α-fluoro-keto species.

Difluoromethylation
Difluoromethylation generates the difluoromethyl group of reagents through nucleophilic addition and radical functionalization of (C–H) bonds. The difluoromethyl group (R-CF2H) has garnered much attention in drug, agrochemical, and material research since it is isoteric to a carbinol group (CH2OH).

Trifluoromethylation
Trifluoromethylation is a rapidly growing field in chemical research that has interfaced elegantly with catalysis in crafting new chemical methodologies for placing trifluoromethyl groups onto molecules.
Perfluoroalkylation
Perfluoroalkylation is the reaction of a nucleophilic perfluoroalkyl group with alkyl, alkenyl, and aryl halides or carbonyl compounds. The stability of perfluoroalkyl reagent groups makes them appealing in a variety of applications such as cross-coupling with allyl phosphates.
Visit our document search for data sheets, certificates and technical documentation.
Related Articles
- Selectfluor™ (1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate), or F-TEDA) is a user-friendly, mild, air- and moisture-stable, non-volatile reagent for electrophilic fluorination.
- (Phen)Cu-CF3 (cat. #L510009) is an easily handled, thermally stable, single-component reagent for the trifluoromethylation of aryl iodides.
- TADDOL chiral auxiliaries find diverse applications in asymmetric synthesis, catalysis, and polymerization.
- The prevalence of organofluorine compounds in industry and drug design necessitates the ability to introduce C–F bonds to molecules.
- Fluoroamidinium salts advance acid halogenation with greater stability and no oxazolones conversion compared to chlorides.
- See All (15)
Find More Articles and Protocols
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email [email protected]
or call +1 (800) 244-1173
Additional Support
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?