Bulk Harvest Release Testing for Vaccines
All biologics, including vaccines, must undergo biosafety testing to meet regulatory requirements. Our long history of supporting the development, manufacture, and commercialization of safe biological drugs and vaccines makes us the partner of choice for companies looking to accelerate vaccines to market by utilizing efficient and robust biosafety testing approaches.
We offer cGMP-compliant bulk harvest release testing support for a variety of vaccine types, including:
- Inactivated / Live-attenuated vaccines
- Vector based vaccines
- Nucleic acid vaccines
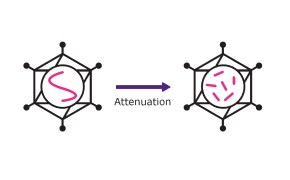
Inactivated / Live-attenuated vaccine
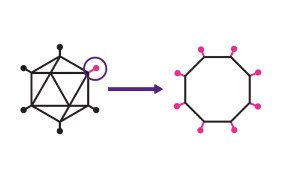
Vector-based vaccine

Nucleic acid vaccine
We take an innovative approach to the development of new, rapid methods for biosafety testing. Many of our GMP compliant assays can be conducted under biosafety level 3 containment, enabling testing for live virus samples (such as those relevant to the production of inactivated viral vaccines and challenge agents) and the execution of viral infectivity assays.
In addition, as part of our extensive range or product characterization services, our potency assays are customized, using platform methods where possible to speed development, based on the expected mode of action of the product.
Related Product Resources
To accelerate your own vaccine to market, use our reply form and get in touch today.
Rapid Biosafety Testing Services Supporting Vaccine Development |
|---|
To continue reading please sign in or create an account.
Don't Have An Account?