Chloroquine Phosphate HPLC Assay & Impurity Profiling
Introduction
A rapid, accurate, and simple method was implemented for the total chromatographic purity analysis of Chloroquine Phosphate by High Performance Liquid Chromatography with a Diode Array Detector. The experimental conditions follow guidelines, with minor modifications, from the USP43-NF38 monograph methods for Chloroquine Phosphate Assay and Impurity Profiling. Chloroquine Phosphate, Chloroquine Related Compound A, Chloroquine Related Compound D, Chloroquine Related Compound E, Chloroquine Related Compound G, Hydroxychloroquine, and Phenol can be resolved with baseline separation within 16 minutes using an Ascentis Express C18 column (250 x 4.6 mm, 5 µM). A 1.4 g/L Dibasic sodium phosphate solution (pH 3.0) in water: 0.4% triethylamine in methanol (30:70 v/v) were employed as the mobile phase for the isocratic elution. Under applied conditions, system suitability criteria are met, and the method demonstrates good resolution/selectivity, reproducibility, and sensitivity.

Chloroquine Phosphate

Hydroxychloroquine sulfate

Phenol

Chloroquine Related Compound A

Chloroquine Related Compound D

Chloroquine Related Compound E

Chloroquine Related Compound G
| Experimental Conditions | |||
|---|---|---|---|
| Column | Ascentis Express C18 column (25cm x 4.6mm, 5µM) | Injection volume | 20 µL |
| Detection | UV @ 260 nm (analytical flow cell; 10 µL) | Flow rate | 1 mL/min |
| Buffer | Dissolve 1.4 g of K2HPO4 in 1000 mL Milli-Q water and adjust to pH 3.0 using H3PO4. | Temperature | Column: 30 °C Autosampler: 16 °C |
| Mobile phase | Buffer and 0.4% triethylamine in Methanol (30:70) v/v. | Pressure | 237 bar |
| Diluent | Mobile Phase | ||
| Test solution | Dissolve 50 mg of Chloroquine Phosphate in 25 mL mobile phase (2.0 mg/mL). | ||
| System suitability solution (SST) | Dissolve 5.0 mg of each Chloroquine Phosphate, Phenol, Hydroxychloroquine, Chloroquine Phosphate Related Compound A, Chloroquine Phosphate Related Compound D, Chloroquine Phosphate Related Compound E, and Chloroquine Phosphate Related Compound G into 25 mL mobile phase. Then, further take 1.0 mL of this solution and dilute to 100.0 mL using diluent (5.0 µg/mL). | ||

Blank
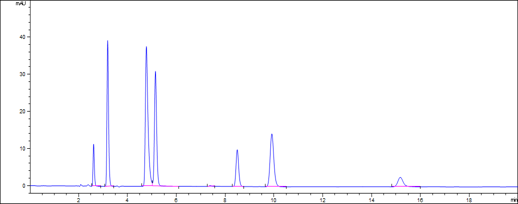
System Suitability Solution (SST) (Standard)

Chloroquine phosphate (Test solution)
Specificity (System Suitability Solution) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Peaks | Compound | Retention Time (min) | RRT | RRT Reference per USP43-NF38 | Resolution | Requirement per USP43-NF38 | Theoretical plates | Tailing factor |
| 1 | Phenol | 2.60 | 0.26 | 0.2 | - | 11484 | 1.1 | |
| 2 | Chloroquine related compound G (RCG) | 3.18 | 0.32 | 0.27 | 5.4 | 11444 | 1.2 | |
| 3 | Chloroquine related compound D (RCD) | 4.77 | 0.48 | 0.42 | 10.5 | 10437 | 1.6 | |
| 4 | Hydroxychloroquine sulfate | 5.14 | 0.52 | 0.49 | 2.2 | 16090 | 1.1 | |
| 5 | Chloroquine related compound A (RCA) | 8.49 | 0.86 | 0.73 | 18.8 | 28913 | 1.1 | |
| 6 | Chloroquine Phosphate | 9.91 | 1.00 | 1.0 | 6.0 | > 2.0 (to RCA) | 20259 | 1.1 |
| 7 | Chloroquine related compound E (RCE) | 15.18 | 1.53 | 1.5 | 15.9 | 23609 | 1.2 | |
Repeatability (System Suitability Solution) | ||||
|---|---|---|---|---|
| Peaks | Compound | Area Response (n=3) | Standard Deviation | RSD (%) |
| 1 | Phenol | 42.42 | 0.13 | 0.3 |
| 2 | Chloroquine related compound G (RCG) | 178.92 | 1.12 | 0.6 |
| 3 | Chloroquine related compound D (RCD) | 283.39 | 0.90 | 0.3 |
| 4 | Hydroxychloroquine sulfate | 195.04 | 0.64 | 0.3 |
| 5 | Chloroquine related compound A (RCA) | 75.60 | 0.22 | 0.3 |
| 6 | Chloroquine Phosphate | 152.47 | 0.70 | 0.5 |
| 7 | Chloroquine related compound E (RCE) | 37.97 | 1.57 | 4.1 |
To continue reading please sign in or create an account.
Don't Have An Account?