Purification of Histidine-Tagged Proteins Secreted into Eukaryotic Cell Culture Supernatants Using HisTrap™ Excel
HisTrap excel 1 mL and 5 mL columns are ready-to-use IMAC columns prepacked with Ni Sepharose excel. The design of the columns in combination with the specific properties of the medium enables fast and convenient purification of histidine-tagged proteins secreted into eukaryotic cell culture supernatant. The special type of filters in the top and bottom of the columns makes it possible to directly load cell-free, unclarified samples on the columns without causing back-pressure problems. This feature saves time, which helps to prevent degradation and thus loss of sensitive target proteins.
HisTrap excel columns are made of biocompatible polypropylene that does not interact with biomolecules. The columns are delivered with a stopper on the inlet and a snap-off end on the outlet. HisTrap excel columns cannot be opened or refilled.
The columns can be operated with either a peristaltic pump or chromatography systems such as ÄKTA systems. For easy scaling up, two HisTrap excel columns can simply be connected in series.
Appendix 1 (Characteristics of Ni Sepharose, Ni Sepharose excel, TALON Superflow, and uncharged IMAC Sepharose products) for the main characteristics of HisTrap excel.
Figure 3.32.HisTrap excel allows direct purification of cell-free, unclarified samples; it is available as 1 mL and 5 mL prepacked columns.
Sample and buffer preparation
Refer to Purification of histidine-tagged proteins secreted into eukaryotic cell culture supernatants using Ni Sepharose excel earlier in this chapter for a general procedure for sample and buffer preparation.
Purification
- Fill the pump tubing with distilled water. Remove the stopper and connect the column to the chromatography system or the laboratory pump “drop-to-drop” to avoid introducing air into the column. Make sure that the connector is tight to prevent leakage.
- Remove the snap-off end at the column outlet.
- Wash out the ethanol with 5 column volumes of distilled water at a flow rate of 1 mL/min.
- Equilibrate the column with at least 5 column volumes of binding buffer. Recommended flow rates are 1 to 4 mL/min (1 mL column) and 5 to 20 mL/min (5 mL column).
- Load the sample. For best results, use a flow rate of 1 to 4 mL/min (1 mL column) or 5 to 20 mL/min (5 mL column) during sample application.
- Wash with 20 column volumes of wash buffer. Maintain a flow rate of 1 mL/min (1 mL column) or 5 mL/min (5 mL column) for washing.
- Elute with elution buffer using a one-step procedure. Five column volumes of elution buffer is usually sufficient. Alternatively, a linear elution gradient (10 to 20 column volumes) may give higher purity, at the expense of lower target protein concentration. Maintain a flow rate of 1 mL/min (1 mL column) or 5 mL/min (5 mL column) for elution.
Purification at low temperatures increases the sample and buffer viscosity, leading to increased back pressure. If necessary, decrease the flow rate.
For A280 measurements, use the elution buffers as blanks. If imidazole needs to be removed, use a desalting column (Chapter 11, Desalting/buffer exchange and concentration). Low-quality imidazole will give a significant background absorbance at 280 nm.
Application example
Purification of protein secreted into insect cell culture medium
In this example, purifications of a protein secreted into insect cell culture medium were compared as follows: A histidine-tagged truncated hemagglutinin ((his)6-HA) expressed in High Five™ insect cells and secreted into insect cell culture medium was applied onto HisTrap excel 5 mL and HisTrap FF crude 5 mL columns (the latter prepacked with Ni Sepharose 6 Fast Flow).
Figure 3.33 illustrates the difference in performance between HisTrap excel and HisTrap FF crude. Total protein yield eluted from the HisTrap excel column was estimated to be 12.7 mg, comprising approximately 8.9 mg of the protein of interest, whereas the amount of protein eluted from HisTrap FF crude was too low to be quantitated. In addition, HisTrap excel retained its blue color after purification whereas HisTrap FF crude did not, further demonstrating that HisTrap excel resists nickel ion stripping. This comparative study clearly demonstrates the advantages of using HisTrap excel when isolating histidine-tagged proteins from a cell culture medium that causes metal ion stripping.
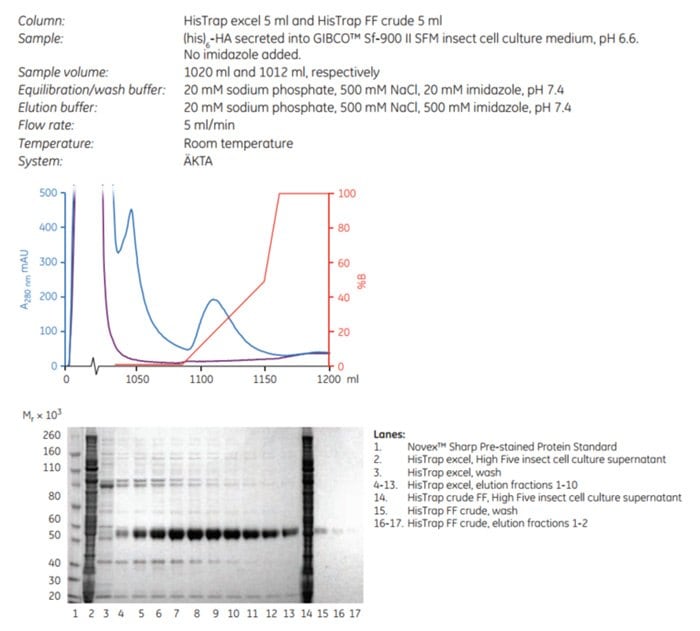
Figure 3.33.(A) Comparative purification of (his)6-HA in insect cell culture supernatant using HisTrap excel (blue) and HisTrap FF crude (purple); (B) SDS-PAGE analysis of elution fractions from the same purifications. The SDS-PAGE gel (reducing conditions) was stained with SimplyBlue™ SafeStain and analyzed with Gel Doc™ XR+ System. We would like to thank Dr. Linda Lua and members of UQ Protein Expression Facility, the University of Queensland, Brisbane, Australia, for performing the application work on (his)6-HA.
To continue reading please sign in or create an account.
Don't Have An Account?