Performing a Separation of DNA Binding Proteins with Cytiva Products Based on Heparin
DNA binding proteins form an extremely diverse class of proteins sharing a single characteristic, their ability to bind to DNA. Functionally the group can be divided into those responsible for the replication and orientation of the DNA such as histones, nucleosomes and replicases and those involved in transcription such as RNA/DNA polymerases, transcriptional activators and repressors and restriction enzymes. They can be produced as fusion proteins to enable more specific purification (page 42, GST fusion proteins), but their ability to bind DNA also enables group specific affinity purification using heparin as a ligand. Heparin is a highly sulphated glycosaminoglycan with the ability to bind a very wide range of biomolecules including:
- DNA binding proteins such as initiation factors, elongation factors, restriction endonucleases, DNA ligase, DNA and RNA polymerases.
- Serine protease inhibitors such as antithrombin III, protease nexins.
- Enzymes such as mast cell proteases, lipoprotein lipase, coagulation enzymes, superoxide dismutase.
- Growth factors such as fibroblast growth factor, Schwann cell growth factor, endothelial cell growth factor.
- Extracellular matrix proteins such as fibronectin, vitronectin, laminin, thrombospondin, collagens.
- Hormone receptors such as oestrogen and androgen receptors.
- Lipoproteins.
The structure of heparin is shown in Figure 1. Heparin has two modes of interaction with proteins and, in both cases, the interaction can be weakened by increases in ionic strength.
- In its interaction with DNA binding proteins heparin mimics the polyanionic structure of the nucleic acid.
- In its interaction with coagulation factors such as antithrombin III, heparin acts as an affinity ligand.
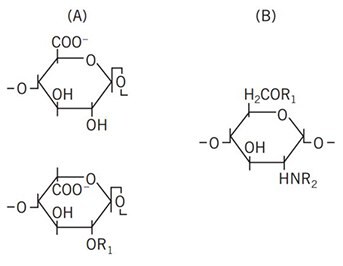
Figure 1. Structure of a heparin polysaccharide consisting of alternating hexuronic acid (A) and D-glucosamine residues (B). The hexuronic acid can either be D-glucuronic acid (top) or its C-5 epimer, L-iduronic acid (bottom).
R1 = -H or -SO3–, R2 = -SO3– or -COCH3
Purification Options
Purification Examples
Figures 2, 3 and 4 show examples of conditions used for the purification of different DNA binding proteins.

Figure 2. Partial purifcation of recombinant HIV-reverse transcriptase on HiTrap Heparin HP.

Figure 3. Partial purification of the recombinant DNA binding Oct-1 protein (courtesy of Dr Gunnar Westin, University Hospital, Uppsala, Sweden) using HiTrap Heparin HP, 5 mL.

Figure 4. scCro8 purification on HiPrep 16/10 Heparin FF.
Performing a Separation
Binding buffers: 20 mM Tris-HCl, pH 8.0 or 10 mM sodium phosphate, pH 7.0
Elution buffer: 20 mM Tris-HCl, 1–2 M NaCl, pH 8.0 or 10 mM sodium phosphate, 1–2 M NaCl, pH 7.0
- Equilibrate the column with 10 column volumes of binding buffer.
- Apply the sample.
- Wash with 5–10 column volumes of binding buffer or until no material appears in the eluent (monitored by UV absorption at A280 nm).
- Elute with 5–10 column volumes of elution buffer using a continuous or step gradient from 0–100% elution buffer.
Modify the selectivity of heparin by altering pH or ionic strength of the buffers. Elute using a continuous or step gradient with NaCl, KCl or (NH4)2SO4 up to 1.5–2 M.
Cleaning
Remove ionically bound proteins by washing with 0.5 column volume 2 M NaCl for 10–15 minutes.
Remove precipitated or denatured proteins by washing with 4 column volumes 0.1 M NaOH for 1–2 hours or 2 column volumes 6 M guanidine hydrochloride for 30–60 minutes or 2 column volumes 8 M urea for 30–60 minutes.
Remove hydrophobically bound proteins by washing with 4 column volumes 0.1% – 0.5% Triton X-100 for 1–2 hours.
Media Characteristics
Chemical Stability
0.1 M NaOH (1 week at +20 °C), 0.05 M sodium acetate, pH 4.0, 4 M NaCl, 8 M urea, 6 M guanidine hydrochloride.
Storage
Wash media and columns with 0.05 M sodium acetate containing 20% ethanol (use approximately 5 column volumes for packed media) and store at +4 to +8 °C.
To continue reading please sign in or create an account.
Don't Have An Account?