3D Cell Culture with Corning Microplates
Accelerate Your Next Discovery
3D cell culture is exploding. Scientists are taking advantage of this evolving technology to generate innovative 3D models never before possible. Corning customers use 3D bioprinting to mimic natural tissues for drug screening. They create organoids to explore the intricacies of various diseases in hopes of delivering more effective treatments. And they produce novel spheroid cell models, along with unique methods of high throughput screening, for more effective drug screening.
Corning is ready, willing, and able to provide the in-depth knowledge, expertise, and hands-on assistance you need to create breakthrough models and deliver what’s next – whatever your 3D approach. Working together, we can rock the science of 3D.
2D or 3D? It’s no longer a question.
Why have so many scientists embraced 3D cell culture? Because cells grown in 3D more closely mimic in vivo behavior in tissues and organs than cells grown in a 2D culture model. 3D cell culture environments create more biologically relevant models for drug discovery which may lead to more accurate outcome predictions, higher success rates for drug compound testing, a faster path to market, and reduced development costs.
Explore 3D Cell Culture Environments
Nobody can predict the body’s precise response to your next discovery. But Corning Life Sciences offers several tools to help you generate in vivo-like conditions for a broad range of cell types, environments, and applications. Our solutions help you create lifelike 3D models that mimic in vivo cell behavior for tissue development, cellular differentiation, and screening assays. These in vitro 3D models enable biologically meaningful experiments and more predictive results to help you accelerate research in challenging and complex areas.
Spheroid Models
Spheroids are simple, widely used multicellular 3D models that form due to the tendency of adherent cells to aggregate. They can be generated from a broad range of cell types resulting in tumor spheroids, embryoid bodies, hepatospheres, neurospheres, and mammospheres.
3D multicellular spheroids can develop metabolic gradients that create heterogeneous cell populations with superior cell-to-cell and cell-to-ECM interactions. 1 They offer a more physiologically relevant model as compared to 2D cell culture and can successfully mimic the microenvironment of a variety of tissue types in disease states.


Scaffold-free Spheroid Models
A scaffold-free environment allows for the self-assembly of cells into suspension colonies without the aid of ECMs or other physical supports.
Hydrogels and ECM Scaffold Models
Hydrogels are a series of cross-linked polymer chains or complex protein molecules of natural (ECMs) or synthetic origin that can be used to encapsulate cells, providing an ideal 3D growth environment.
Solid Synthetic Scaffold Models
Solid scaffolds are porous structures offered in a variety of materials and geometries that mimic 3D microenvironments for a diverse set of cell culture applications.
Corning Solutions for Scaffold-free Spheroid Models
Scaffold-free Spheroid Models
Common scaffold-free methods for generating spheroids include suspension cultures in media, hanging drop methods, or attachment-resistant cell culture surfaces such as Corning Ultra-Low Attachment surface and spheroid microplates. These ready-to-use products offer a biologically inert surface that minimizes cell attachment and promotes the formation of 3D multicellular spheroids employed in cancer research, stem cell biology, and drug screening.
Ultra-Low Attachment Surface
The Corning® Ultra-Low Attachment (ULA) surface is a proprietary, animal-free, covalently bonded hydrogel surface that is hydrophilic and neutrally charged. It minimizes cell attachment, protein absorption, and enzyme activation. The surface is non-cytotoxic and non-degradable. The ULA surface promotes the formation and easy harvesting of anchorage-dependent scaffold-free spheroids and is available in a variety of cell culture formats and configurations (Figure 1).

Figure 1. Tissue Culture-treated surface (left) and spheroid colonies on Ultra-Low Attachment surface (right).
Spheroid Microplates
Corning Spheroid microplates combine the Corning Ultra-Low Attachment surface with an innovative U-shaped well geometry. This easy-to-use, highly reproducible tool is ideal for generating, culturing, and assaying individual uniformly sized 3D multicellular spheroids in the same microplate – with no need for transfer. The microplates feature opaque side walls and a proprietary gridded plate bottom to reduce well-to-well cross-talk and background fluorescence/luminescence for a variety of drug and toxicity screening applications. Corning spheroid microplates are available in 96-, 384-, and 1536-well automation-friendly formats.
They enable direct high throughput screening of spheroids in these microplates, cutting down associated scale-up steps (Figure 2).
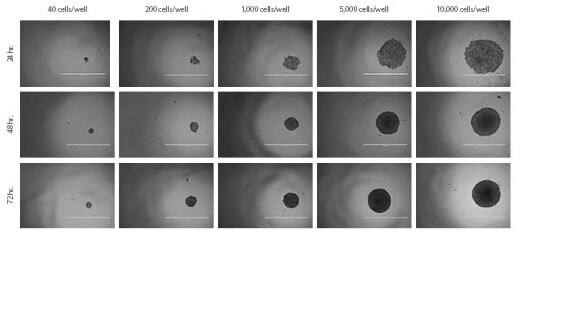
Figure 2. HT-29 Spheroids. Spheroid formation and growth of HT-29 spheroids over time with varying seeding densities.
Scaffold-free Spheroid Model Applications
Corning-powered spheroid models are the first choice in critical and complex research areas.
Stem Cell Research
Corning® spheroid microplates are used to create uniform embryoid bodies from induced pluripotent stem cells (iPSCs) that can be subsequently generated into high purity neural stem cells (NSCs) for the study of potential neural disease treatments (Figure 3).
Corning 96-well Spheroid Microplate Non-treated Microplate

Figure 3. EB Selection in defined medium. Morphology of putative NSCs produced from iPSCs cells by various protocols. Scale bar: 100 µm.
Tumor Biology
3D tumor spheroid models generated with Corning spheroid microplates closely mimic the in vivo tumor microenvironment. These spheroids – grown as either single cultures or more complex co-cultures with other cell types in the tumor microenvironment – offer an opportunity to better predict
the therapeutic efficacy of cancer drug models in oncology drug discovery applications (Figure 4)
Immune Oncology
Corning 384-well spheroid microplates have been successfully employed to evaluate the cytotoxic effect of CAR-T cells on tumor cell spheroids. For example, the KILR™ Cytotoxicity assay (DiscoverX Corp.) combined with KILR-transduced tumor spheroids can be formed, cultured, and assayed directly on the same spheroid microplate (CLS-AN-447).

Figure 4. Live (green) and dead (red) stained 96-hour mono- and co-culture A549 and fibroblast spheroids.Spheroids were exposed to high (34.5 µM) and low (27.6 µM) doses of doxorubicin or vehicle control [no doxorubicin] for 48 hours in Corning 384-well spheroid microplates. Fibroblast (FB) monoculture displayed the most intense live staining upon low dose exposure to doxorubicin, while A549 monoculture showed increased cell death. The 9:1 ratio of FB to A549 cells also displayed a protective effect at the low dose doxorubicin exposure. All cell types displayed significant toxicity after high dose exposure. Images captured using an EVOS® fluorescent microscope at 10X objective. Scale bar = 400 µm.
Hydrogel and ECM Scaffold Model Applications
Hydrogels are made up of a network of highly absorbent, cross-linked polymer chains or complex protein molecules of natural or synthetic origin. They can encapsulate and release a variety of bioactive molecules.2 Due to their high water content, hydrogels closely resemble the tissues in vivo and act as excellent 3D matrices. They can be used either alone or combined with other technologies such as permeable supports. Hydrogels work seamlessly with a variety of microplate formats and automated equipment used in high throughput screening applications.
Corning Solutions for Hydrogels and CM Scaffold Models
Natural (Biological) Hydrogels and ECMs
Hydrogels derived naturally are formed from extracellular matrices (ECM) proteins and contain a variety of endogenous factors including soluble biomolecules and growth factors that can be beneficial for supporting cell viability, cell migration, function, and differentiation. These ECMs are also amenable to matrix degradation and deposition.
Corning® Matrigel® Matrix
The most widely used natural ECM is Corning Matrigel matrix, a reconstituted basement membrane extract from Engelbreth-Holm-Swarm (EHS) mouse tumors. It is rich in laminin, collagen IV, entactin, heparin sulfate proteoglycans, and a number of growth factors, making it ideal to use in applications including cancer and stem cell research. Matrigel matrix is a bioactive matrix which exhibits the mechanical and chemical properties of the in vivo ECM. It offers a dynamic and tunable microenvironment for the cells to grow and develop.3 The ECM components of Matrigel matrix activate cellular responses and pathways that are more physiologically relevant compared to cells grown in 2D surfaces (CLS-AC-AN-245).
Collagen
Corning Collagen Type I is a natural hydrogel commonly found in stromal compartments in the dermis, tendon, and bone. It is effective as a gel and supports in vivo-like 3D growth and differentiation. It provides physiological interactions with receptors to modulate the expression of a variety of genes including those involved in cell invasion, cell sensitivity to anti-cancer drugs, cell proliferation, and cell migration (CLS-AC-AN-245). Collagen I has also been successfully used to culture intestinal, pancreatic, mammary, and salivary organoids.4,5,6
“Matrigel® is a common and important component of the system that provides a scaffold and additional supplementation of signaling cues via basement membrane ligandXu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001; 19:971-974.
Xiaolei Yin, Benjamin E. Mead, Helia Safaee, Robert Langer, Jeffrey M. Karp, and Oren Levy. Stem Cell Organoid Engineering. Cell Stem Cell. 2016 January 7; 18(1): 25-38.s to support cell attachment and survival, as well as organoid formation.”
Synthetic Hydrogels
In cases where bioactive compounds, such as growth factors, can potentially interfere with the specific cell behaviors or responses, synthetic hydrogels are a good choice for 3D cell culture applications. Synthetic hydrogels are biologically inert, pathogen-free, non-natural molecules that offer structural support for a variety of cell types. It is also possible to blend synthetic scaffolds with biological components to create tunable synthetic hydrogels.
Corning® PuraMatrix™ Peptide Hydrogel
This synthetic oligopeptide self assembles into a 3D hydrogel with nanometer-scale fibers. When combined with bioactive molecules, PuraMatrix forms a 3D environment for cells that can be plated on top of the hydrogel to study cell movement or suspended within the matrix for 3D encapsulation applications. The transparent nature of PuraMatrix makes it easy to visualize samples via staining and standard microscopy. This biocompatible format supports the differentiation of a variety of primary and stem cell types including hepatocytes and neural cells.7,8
Hydrogel and ECM Scaffold Model Applications
Organoids
Organoids have become an increasingly popular option for scientists in development and drug discovery as they resemble the composition and functionality of organs. Stem cells and/or organ progenitors from normal or diseased tissue are mixed with Corning® Matrigel® matrix to create mini-organs of the kidney, thyroid, liver, brain, lung, intestine, prostate, and pancreas. Organoids support advancements in the study of organogenesis, disease modeling, and subsequently patient-specific therapies. Matrigel matrix is the most published and optimum hydrogel for organoid research due to its close resemblance to an in vivo environment, providing necessary growth factors, proteins, and the required matrix architecture. Scientists are bioprinting organoids to recreate mini-organs. Biological hydrogels such as Corning Collagen and Corning Matrigel matrix can be used as bio-inks to enable precise positioning and embedding of living cells during printing.

Figure 5. Top image features human rectal organoids from a cystic fibrosis cell source cultured for 10 days in Corning Matrigel matrix. Courtesy of MDI Biological Laboratory. Bottom image features Human liver organoids in Corning Matrigel matrix (perm. L.A. Oosterhoff).
Modeling Cancer
Tumor models using Matrigel matrix replicate the 3D structure of tumors to study formation, progression, invasion, and metastasis of cancer cells and to evaluate potential drug treatments. Cell morphology in a 3D Matrigel matrix culture can also be used to predict and/or stage cancer progression based on behaviors of cell polarization, colony formation, and proliferation. To metastasize, cancer cells must be able to cross a matrix and enter the blood vasculature. Matrigel matrix cell invasion assays can be used to assess the aggressiveness of cancer cells to demonstrate their potential of degrading the ECM proteins by proteases that can lead to metastases.
Angiogenesis
Angiogenic capability is often assessed by the ability of endothelial cells to sprout, migrate, and form vascular tubules. Matrigel matrix formulations allow in vitro modeling of endothelial cell behavior, including survival, apoptosis, tube formation, and invasion. These models are often used to investigate the effects of drugs or small molecules on angiogenesis in vitro. For in vivo evaluation of angiogenic drug compounds, the subcutaneous Matrigel matrix plug assay in mice is one such option.
Corning Matrigel matrix plug assay has become the method of choice for many studies involving in vivo testing for angiogenesis.”
Akhtar N, Dickerson EB, Auerbach R. The sponge/Matrigel angiogenesis assay. Angiogenesis (2002) 5:75-80.

Figure 6.Corning Human Umbilical Vein Endothelial Cells (HUVEC-2) stained with Calcein AM and cultured on Corning Matrigel matrix.
Spheroids vs. Organoids – What’s the difference?

Spheroids and organoids are both multicellular 3D structures. Although this terminology has been used interchangeably, there are distinct differences between the two. An organoid is a collection of organ-specific cell types that develops from stem cells or organ progenitors, which self-organize through cell sorting and spatially restricted lineage commitment in a manner similar to in vivo.9 Multicellular tumor spheroid models were first described in the early 1970s and were obtained by culturing cancer cell lines under non-adherent conditions.10 Tissue-derived tumor spheres and organotypic multicellular spheroids are typically obtained by tumor tissue mechanical dissociation and cutting.11 Tumorospheres are a model of cancer stem cell expansion. Generally, there is a higher order self-assembly in organoids as opposed to spheroid cultures and the former is more dependent on an extracellular matrix for its generation.

Figure 7.Description of cerebral organoid culture system. (A) Schematic of the culture system used to generate cerebral organoids. Example images of each stage are shown: bFGF, basic fibroblast growth factor; hES, human embryonic stem cell; hPSCs, human pluripotent stem cells; RA, retinoic acid. (B) A comparison between organoid and mouse brain structure demonstrates recapitulation of dorsal cortical organization. Immunohistochemistry for neurons (TUJ1, green) and radial glial stem cells (PAX6, red) in a large dorsal cortical region. (C) Sectioning and immunohistochemistry revealed complex morphology with heterogeneous regions containing neural progenitors (SOX2, red) and neurons (TUJ1, green). (D) Low-magnification bright-field images revealing fluid-filled cavities reminiscent of ventricles and retina tissue, as indicated by retinal pigmented epithelium.12
Solid Synthetic Scaffold Models
Solid scaffolds can be made from a broad range of materials to mimic a given 3D microenvironment. Polymers are a common choice for generating solid scaffolds of diverse size, structure, and porosity. They can be fabricated using lithography, electrospinning, bioprinting1 and, in the case of permeable supports, microporous membranes.
Because synthetic scaffolds are devoid of animal-derived materials, they are free of potential pathogens and other issues found with biologic products. For studies where endogenous factors are required to more realistically mimic the cellular in vivo environment, they can be combined with ECMs as a coating to create effective complex matrices for 3D cell culture.2
Solid Synthetic Scaffold Applications
Organotypic Tissue Models and Air-Liquid Interface (ALI) Culture
Organotypic models have been developed for a variety of tissues including skin, liver, stomach, kidney, and lung. They display a realistic micro-anatomy, mimic organ functionality, and offer insight into cell-to cell interactions, making them a valued research tool for drug discovery, regenerative medicine, toxicity testing, and disease modeling. Permeable supports are particularly well suited for growing organotypic models as their design makes it possible to bathe cells both apically and basolaterally or grow epithelial cell models at the air-liquid interface (ALI) and bathe them basolaterally. ALI culture results in more differentiated phenotypes and physiologically relevant models than traditional 2D submerged culture on solid plastic substrates13 (Figure 8).
Corning Solutions at Work
Transwell® and Falcon® permeable supports from Corning are solid synthetic scaffold inserts with microporous membrane bottoms. They are available in multiple formats, pore sizes, and membrane types to cater to a variety of application needs. These cell culture inserts provide a dual compartment system that allows cells to carry out a variety of metabolic activities in a more in vivo-like manner through the exchange of media, nutrients, and other biomolecules. They have been widely used to grow complex, multilayered tissues such as skin, liver, kidney, and human airway epithelia. Permeable supports are valuable tools for emulating aspects of normal physiology when growing complex tissues, co-culturing cells, and studying cellular movement. When used in combination with ECMs, they can be used to produce cancer invasion models.
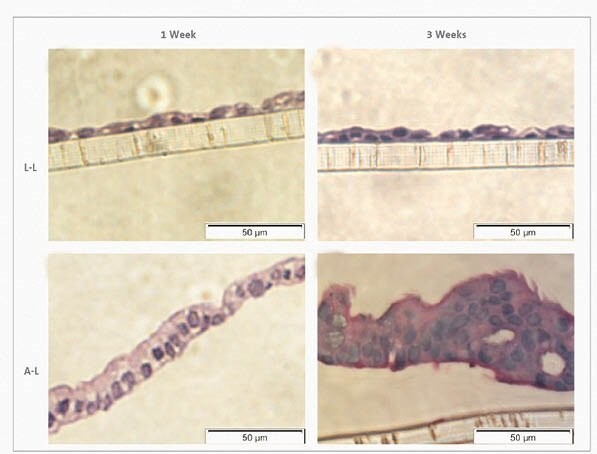
Figure 8. Haematoxylin-eosin stain of Calu-3 cells at the L-L and A-L interfaces after 1 week and 3 weeks in culture. Haematoxylin-eosin stain at the L-L interface revealed a flat cell monolayer (A, B). At the A-L interface, pseudostratified mucociliary epithelium morphology was formed (C, D).
Solid Synthetic Scaffold Applications (continued)
Bioprinting
Bioprinting fabricates a 3D tissue-like construct, layer-by-layer using cells, spheroids, or organoids suspended in a bio-ink.14 3D bioprinting has been used for the generation of multilayered skin, bone, liver, and cartilage tissue models in research, toxicology, and drug-screening studies. Bioprinting makes it possible to reproduce structural features seen in vivo and explore the cell-to-cell relationships that affect tissue functionality.15
Transwell® permeable supports have been successfully used as a bioprinting substrate for a variety of tissue models, including liver and kidney. The microporous surface and the compartmentalized design of the inserts provide an excellent substrate for prolonged cell culture of the bioprinted tissue in an in vivo-like environment that can also be subsequently used in drug discovery testing and disease modeling (Figure 9).

Figure 9. 3D human liver tissue bioprinted on Corning Transwell permeable supports. Image courtesy of Organovo.
Motility Models
The movement of cells from one area to another in response to a chemical signal, is central to a variety of cell functions such as cell differentiation, wound repair, embryonic development, angiogenesis, and tumor metastasis.
Corning permeable supports offer a simple in vitro 3D method for studying this movement. They can be used alone in culture or coupled with ECMs, such as Corning® Matrigel® matrix or Corning spheroid microplates, to study a wide variety of cell types and movement mechanisms.
Recent advances in the development of a light-blocking membrane, known as Corning FluoroBlok™, have further simplified this process by eliminating the need to swab away non-migrating cells after a migration event. Fluorescently labeled cells present in the top chamber of the insert are shielded from bottom-reading fluorescence plate readers and microscopes. After labeled cells migrate through the membrane, they are easily detected by a bottom-reading fluorescence plate reader or microscope, thereby eliminating manual cell counting and additional processing steps. This non-destructive detection method enables both kinetic and endpoint migration and invasion assays. Corning offers a FluoroBlok light-blocking membrane in several insert formats designed for the study of cell movement (Figures 10 and 11).

Figure 10. Corning® FluoroBlok™ Insert System
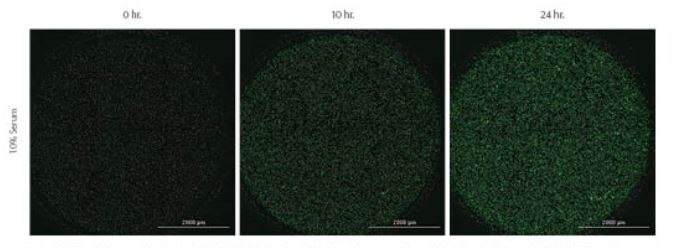
Figure 11. Kinetic images taken of Green-labeled MDA-MB-231 cells labeled prior to experiment with CellTracker™ Green CMFA dye. 10% or 0% serum was used as positive and negative chemoattractant controls, respectively. Images captured using GFP imaging filter cube and a 4X objective.

Figure 12. Immune Oncology Model Schematic
- Day 0
Seed MOCKII/MDR1 into HTS 96-well Transwell® inserts
- Day 4
Seed LN229 cells into Spheroid microplate
- Day 5
Combine HTS 96-well Transwell inserts into a Spheroid microplate and expose to drugs for 2 hours. After drug incubation, remove Transwell and test for monolayer integrity. Culture spheroids for 2 additional days.
- Day 7
Assay spheroids with CellTiter-Glo® 3D

Figure 13. 3D Glioma Spheroid Cytotoxicity Assay. LN229 cytotoxicity with, or without, blood brain barrier (BBB) surrogate.Percent viability of LN229 spheroids 48 hours after 2-hour 250 µM drug exposure through Transwell inserts with, or without, a BBB. Viability was assessed by normalizing buffer controls to 100% viability. Data shown the average of 3 independent studies, N = 30 with 1-way ANOVA with Bonferroni’s post-test. *** = p<0.0001.
Corning Tools Work Best Because They Work Together
Many Corning 3D tools can be used together to study physiological mechanisms. For example, Corning® Matrigel® matrix or Collagen, work with our permeable supports, as well as with multiwell plates and spheroid microplates for organoid and cancer biology (CLS-AN-464).
One novel model that combines these 3D tools involves using Corning spheroid microplates with Corning HTS Transwell-96 well permeable supports for the study of a variety of complex tumoricidal events. Another unique model is high throughput brain tumor testing which traditionally requires testing for cytotoxic compounds in addition to testing for a drug’s ability to pass the blood brain barrier (BBB). By combining the Corning spheroid microplates and Corning HTS Transwell-96 well permeable supports, you can test tumor cytotoxicity and BBB permeability – all in one easy to use, 3D, high throughput assay (Figures 12 and 13, CLS-AN-505).
A Commitment to 3D and You
Corning Life Sciences has more than 30 years of experience delivering real 3D innovations – products, platforms, protocols, applications, education, and support. Our experts work with you to overcome your most complex 3D challenges. Our solutions are designed to help you accelerate research in critical areas such as cancer biology, tissue engineering, and regenerative medicine – to bring safe, effective drugs and therapies to market in less time with greater confidence. Find out how Corning can help you create more in vivo-like 3D models, conduct more biologically relevant experiments, and better predict how your next discovery will behave in the real world.
References
To continue reading please sign in or create an account.
Don't Have An Account?