Applications of Percoll In Blood Cells
Blood cells
The entire spectrum of cell types present in blood can be resolved on preformed gradients of Percoll. The method described by Pertoft et al. (55) (Fig 19) utilizes both rate zonal (separation by size) and isopycnic (separation by density) techniques. Diluted blood was layered on top of a preformed self-generated gradient and centrifuged for 5 min at 400 × g, during which time the thrombocytes or platelets (which are appreciably smaller than the other cells present) did not penetrate into the gradient.
The plasma layer containing the thrombocytes was removed and replaced by saline, and centrifugation was continued at 800 × g for 15 min, resulting in isopycnic banding of mononuclear cells (lymphocytes and monocytes), polymorphonuclear cells and erythrocytes. The position and densities of the banded cells were monitored using Density Marker Beads in an identical gradient contained in a second centrifuge tube.
Although the above method demonstrates the utility of Percoll for fractionating whole blood, most blood cells can be appreciably enriched using a simple step gradient. A simple step gradient often gives acceptable yields and purity for downstream processing. The Application Tables below contain a number of examples of purification of blood cells and other cell types using different types of Percoll gradients.
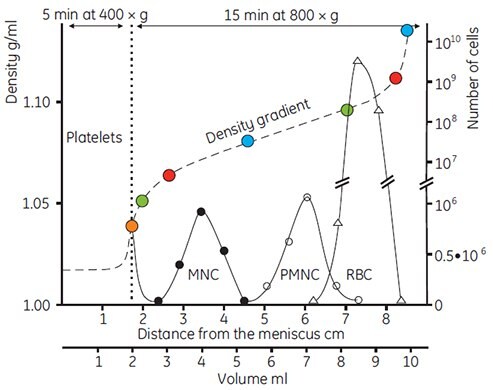
Figure 19.Separation of human blood cells in a gradient of Percoll. The tubes were filled with 10 ml of 70% (v/v) Percoll in 0.15 M NaCl (p=1.086 g/ml), and the gradient performed by spinning in a 14° angle rotor at 20 000 × g for 15 min. Two ml of gradient material was removed from the bottom of the tube using a syringe, and 1 ml of heparinized blood diluted with 1 ml of 0.15 M NaCl was layered on top of the gradient. Centrifugation was carried out as indicated. Densities were monitored using Density Marker Beads. MNC = Mononuclear cells, PMNC = Polymorphonuclear cells, RBC = Red blood cells (55, reproduced by kind permission of the authors and publisher).
The following tables were compiled to assist the researcher in selecting references most likely to contain relevant information regarding use of Percoll for a particular cell or tissue type.
| Lymphocytes | |||||
|---|---|---|---|---|---|
| Species | Gradient type | Tissue type | Comments | Downstream application | Ref. # |
| human | continuous | blood | Percoll density centrifugation resulted in significant downregulation of L-selectin surface reactivity. | Immunoflourescence | 891 |
| human | continuous | tonsil | A Percoll density gradient was used for separation of large (low density) in vivo activated cells from small (high density) resting cells. | cell culture, FACS, granulocytemacrophage colony stimulating factor (GM-CSF) assays, and Northern blots | 892 |
| human | continuous | spleen, tonsil | Large B lymphocytes from tonsils (in vivo activated cells) obtained by Percoll gradient centrifugation displayed higher IL-4R levels than resting cells. | cell culture, Northern blots, FACS | 893 |
| human | continuous | peripheral blood | Percoll was used to separate proliferating form nonproliferating cells. | tritiated thymidine incorporation | 11 |
| human | continuous | tonsil, peripheral blood | This procedure yielded > 90% viable cells and has proved quite helpful in renewing overgrown cultures. | proliferation and cytotoxicity assays | 16 |
| human | continuous | blood | Percoll was used to separate monocytes from lymphocytes. | cell culture, coagulation activity, immunoradiometric assays | 40 |
| human | discontinuous (3-layer) | intestine | Lymphocytes were enriched in the interface between 66.7 and 44% Percoll. Further purification was performed using magnetic beads. | flow cytometric analysis, immunoperoxidase procedure, cell culture | 894 |
| human | discontinuous (6-layer) | peripheral blood | Percoll was used to separate large granular lymphocytes (LGL) from peripheral mononuclear cells. | detection of CD5LOW+ in the LGL population | 895 |
| human | discontinuous | tonsil | Percoll gradient was used for the separation of small (high density) and large (low density) cells. | cell culture, apoptosis assays, immunoassay for G-CSF, bioassay for GM-CSF, Northern blot analysis | 896 |
| human | discontinuous | intestine | proliferation assays, measurement of cytotoxicity, H1 receptor binding studies | 897 | |
| human | discontinuous | peripheral blood | Percoll was used for the isolation of low density cells. | FACS, immunoflourescence, nonspecific esterase staining | 898 |
| human | discontinuous | peripheral blood | Lymphoctes were recovered from low density Percoll fractions. | suppression of NK-cell proliferation by freshly isolated monocytes | 899 |
| human | discontinuous | tonsil | Percoll was used to isolate follicular dendrite cells (FDCs). | cell sorting, B cell proliferation by FDCs | 900 |
| human | discontinuous | bone marrow | Percoll was used to isolate leukemic cells from bone marrow. | establishment of a leukemic cell line | 901 |
| human | discontinuous | peripheral blood | After separation on Percoll, a virtually pure population of activated cells was obtained, as estimated by the presence of the 4F2 marker and of the transferrin receptor. | immunoflourescence and assay of phospholipid metabolism | 902 |
| human | discontinuous (1-step) | blood | Lymphocyte purity was > 99% and the population of monocytes was enriched 82 to 90%. | induction and assay of lymphokine (IL-2)-activated killer (LAK) cell activity | 903 |
| human | discontinuous (4-layer) | blood | Percoll was used for separation of large granular lymphocytic (LGL) cells from T cells. | Giemsa staining, cell activation with interleukin-2 (IL-2) | 904 |
| human | discontinuous (4-layer) | tonsil | Percoll was used for B cell enrichment. | flow cytometry | 905 |
| human | discontinuous (5-layer) | blood | Large granular lymphocytes (LGL) were collected from the low density fractions, whereas T cells were located in the higher density bottom fraction. | FACS, cell culture, cytotoxicity assays | 906 |
| human | discontinuous (5-layer) | blood | Monocytes were purified up to 90% and lymphocytes to > 99%. | cell counting (hemocytometer) and cell culture assays | 69 |
| human | discontinuous (7-layer) | peripheral blood | cytotoxicity assay, flow cytometry analysis, and complement-dependent lysis | 907 | |
| human | selfgenerating | peripheral blood | Percoll was used to separate viable and nonviable cells. Yields were slightly higher and erythrocyte contamination was slightly lower with Percoll than with Ficoll- Isopaque. | cytotoxicity assays | 83 |
| canine | continuous | blood | Percoll was used for enrichment and depletion of antibody-positive cells. | reverse hemolytic plaque assay and cell-mediated lympholysis | 908 |
| canine | discontinuous (4-step and 2-layer) | whole blood | A final sedimentation of purified lymphocytes through a 45/50% Percoll gradient concentrated natural killer (NK) activity into a single band of lymphocytes. | measurement of NK activity | 909 |
| mouse | continuous (3-layer) | intestine | Enrichment increased from 44.1% (single filtration) to 52.4% (multiple filtration) after nylon wool filtration, and from 70.3% (single filtration) to 82.8% (multiple filtration) after Percoll fraction. | flow cytometry | 910 |
| mouse | continuous (5-layer) | spleen | Percoll was used for separation of virgin and memory T cells. | cell proliferation assays, FACS | 911 |
| mouse | discontinuous (3-layer) | spleen | Percoll was used for separation of B cells. | protein phosphorylation assay | 912 |
| mouse | discontinuous (3-layer) | intestine | Percoll was used for isolation of intestinal intraepithelial lymphocytes (IEL). | DNA analysis by flow cytometry, mRNA-cDNA dot blots, PCR | 913 |
| mouse | discontinuous (4-layer) | spleen | Percoll was used for isolation of small, resting B cells. | cell cycle analysis by flow cytometry | 914 |
| bovine | discontinuous | mammary | Purified cells were > 80% pure. | Wright´s Giemsa staining, cell culture | 915 |
| Monocytes | |||||
|---|---|---|---|---|---|
| Species | Gradient type | Tissue type | Comments | Downstream application | Ref. # |
| human | discontinuous (minigradient) | peripheral blood | With the Percoll minigradient, cells could be obtained in 90 to 100% from the patients at all time points after bone marrow transplant (BMT). | cytogenic analysis | 916 |
| human | continuous | blood | The isolated mononuclear leukocyte (MNL) fraction contained > 80% cells giving a positive reaction for a-naphthyl acetate esterase ( a-NAE). | cell culture | 917 |
| human | continuous | peripheral blood | Percoll was used to isolate monocytes with > 85% purity and > 95% viability. | cell culture with cytokines | 918 |
| human | continuous | blood | Percoll has proved very practical for the separation of monocytes from blood and of macrophages from ascites and synovial fluids. | cell culture | 34 |
| human | continuous | blood | Percoll gradients were used for the separation of monocytes from lymphocytes. | cell culture | 40 |
| human | continuous | blood | A one-step procedure was used for obtaining a high-yield suspension of monocytes of 20% purity, which does not require washing before cultivation. A two-step method gave better than 90% pure monocytes at a lower yield. | cell counts, Fc-receptor presence and phagocytosis assays | 57 |
| human | continuous | peripheral blood | MNL were separated into two fractions with Percoll: one consisting mostly of monocytes and the other lymphocytes. | fungal (Coccidioides immits) killing assay | 919 |
| human | discontinuous | blood | Monocyte purity was 95%. | cell culture | 920 |
| human | discontinuous | whole blood | Percoll gradient was used for enrichment of hematopoietic progenitor cells. | assay for colony formation | 921 |
| human | discontinuous | blood | RNA isolation, Northern blot analysis and RT-PCR | 922 | |
| human | discontinuous | bone marrow | DNA hybridization studies | 923 | |
| human | discontinuous (1-layer) | blood | Lymphocyte purity was > 99% and the population of monocytes was enriched 82 to 90%. | induction and assay of lymphokine (IL-2)-activated killer (LAK) activity | 903 |
| human | discontinuous (1-layer) | blood | PMN recovery was > 90% and RBC contamination < 5%. | Northern blot analysis | 924 |
| human | discontinuous (1-layer) | peripheral blood | Monocytes were ≥ 95% pure. | Northern blot analysis, nuclear runoff experiments, S1 protection assay | 925 |
| human | discontinuous (4-layer) | peripheral blood | Cells obtained from the 65% to 75% interface were 99% granulocytes. | analysis and Western blot analysis genomic DNA isolation and PCR | 926 |
| human | discontinuous (1-layer) | peripheral blood | With the 1-step gradient, the purity of the monocytes was 93 to 96%. | Giemsa staining and cell culture | 927 |
| human | discontinuous (5-layer) | peripheral blood | Percoll-isolated monocyte/ macrophages as identified by Wright-Giemsa stain. | interactions between monocyte/macrophage and vascular smooth muscle cells | 928 |
| human | discontinuous (5-layer) | blood | Monocytes were purified up to 90% and lymphocytes to 99% purity. | cell recovery counting and cell culture assays | 69 |
| human | discontinuous | peripheral blood | cell enumeration with Coulter counter, RNA isolation, and Northern blot analysis | 929 | |
| equine | discontinuous (1-layer) | peripheral blood | All MNCs were recovered on Percoll gradients without any neutrophil contamination. | cell recovery assays | 930 |
| Erythrocytes | |||||
|---|---|---|---|---|---|
| Species | Gradient type | Tissue type | Comments | Downstream application | Ref. # |
| human | continuous | whole blood | Percoll was used for separating young and old erythrocytes. | immunoflourescence analysis of complement receptor type 1 (CR1) and CD59, proteolytic cleavage of CR1 in vivo. | 931 |
| human | continuous | blood | Percoll was used to separate Plasmodium falciparum-parasitized erythrocytes from nonparasitized erythrocytes. | isolation of erythrocyte membranes lipid peroxidation, vitamin E and transmembrane reducing system analysis | 932 |
| human | continuous | blood | A rapid method for the age fractionation of human erythrocytes by Percoll density gradient centrifugation was described. | flame photometry, enzyme assays | 77 |
| human | discontinuous (4-layer and 8-layer) | blood | A rapid method using Percoll to fractionate erythrocytes according to age was described. | analysis of the decline of enzymatic activity in aging erythrocytes | 933 |
| human | discontinuous (4-layer) | blood | ELISAs, proteolytic digestion of membranes | 934 | |
| human | discontinuous (4-layer) | blood | The position of Density Marker Beads (Cytiva) was used to collect cells with densities < 1.00 g/cm3 or > 1.119 g/cm3. | yield stress experiment: a sensitive index of cell: cell adhesion of deoxygenated suspensions of sickle cells | 935 |
| human | discontinuous | blood | Percoll gradient was used to separate erythrocytes into 4 density fractions. | platelet-activating factor (PAF) acetylhydrolase activity and membrane fluidity | 936 |
| human | discontinuous | blood | Erythrocytes loaded with L-asparaginase using a hypotonic dialysis process were separated into eight fractions. | L-asparaginase activity | 937 |
| human | discontinuous | blood | Discontinuous gradient of the range 1.080 to 1.115 g/cm3 with each layer differing in density by 0.005 g/ml produced nine cell fractions. | enzyme assays | 66 |
| human | discontinuous (5-layer) | blood | study of RBC deformability and cell age | 938 | |
| human | discontinuous (8-layer | blood | Percoll was used for density separation of RBC loaded with inositol hexaphosphate (IHP) by reverse osmotic lysis. | haemoglobin distribution, distribution of IHP concentrations | 939 |
| human | discontinuous (9-layer) | blood | A detailed comparison between two cell-loading techniques for inositol hexaphosphate was performed by monitoring the RBC distribution patterns on Percoll density gradients. | oxygen affinity, hematological parameters and organic phosphate content measurements | 940 |
| Mastomys natalensis | continuous | blood | Percoll was used to separate Plasmodium berghei-paraitized erythrocytes from non parasitized cells. | cAMP level in RBCs | 941 |
| mouse | continuous (self-forming) | blood | Fractionation of RBC yielded five distinct populations that maintained their densities upon recentrifugation in a second gradient. | transbilateral movement and equilibrium distribution of lipid | 942 |
| mouse | continuous | peripheral blood | Percoll was used for density gradient separation of chemicallyinduced erythrocytes. | fixing, staining and flow cytometric analysis of micronucleated polychromatic (MPCE) and micronucleated nonchromatic (MNCE) erythrocytes | 943 |
| mouse | discontinuous | peripheral blood | Erythrocytes were contaminated with only 0.001% nucleated cells. | glucose phosphate isomerase (GPI) assay | 944 |
| rat | discontinuous | whole blood | Percoll was used to separate Plasmodium berghei-infected RBCs. | oxygen dissociation analysis | 945 |
| rabbit | discontinuous (7-layer) | blood | Rabbit red blood cells were reproducibly fractionated into populations of various stages of maturation. | measurement of cytosolic protease activities | 946 |
| trout | discontinuous | blood | The gradient in the region of 45 to 65% Percoll produced three red cell fractions which is due to multiplicity of haemoglobin components. | antioxidant enzyme activities and membrane fluidity analysis | 947 |
| Natural Killer (NK) cells | |||||
|---|---|---|---|---|---|
| Species | Gradient type | Tissue type | Comments | Downstream application | Ref. # |
| human | discontinuous | peripheral blood | The Percoll (preculture) step facilitated the density separation of resting cells from larger lymphocytes. | NK- and T-cell activation, immunoflourescence | 948 |
| human | discontinuous | blood | K562 cells which adhere to NK cells were separated together. Enrichment of NK cells was 71.3%. | cytotoxicity studies, morphological characterization | 61 |
| human | discontinuous (2-layer) | peripheral blood | The low density fraction (42.5 to 47.5% Percoll) which showed a 4-fold enrichment in NK activity was used. | NK activity and kinetic constant determinations, measurement of the effect of divalent cations on NK activity, and effect of ATP on NK cell-surface markers | 949 |
| human | discontinuous (6-layer) | blood | Further purification using magnetic beads resulted in a pure preparation. | cytotoxic assay | 950 |
| human | discontinuous (8-layer) | peripheral blood | Recovery was > 80% while viability, as judged by trypan blue exclusion, was > 95%. | NK cell stimulatory effect, phenotype evalution by immunoflourescence | 951 |
| mouse | discontinuous (3-layer) | lung | The cells at the 50/55% interface were the richest in NK cell activity. | adoptive transfer to reconstitute NK activity in NKdepleted mice | 952 |
| mouse | discontinuous (6-layer) | spleen | NK cells were enriched in the lower density Percoll fraction, while natural cytotoxic T cells (NCT) were distributed between both higher and lower density fractions. | cytotoxicity of NK cells was measured | 953 |
| mouse | discontinuous (6-layer) | liver | All NK activity was above 1.08 g/ml density. Interfaces at 1.04 and 1.06 gave a 2× enrichment of NK progenitors. | PCR, Western blot analysis, and cytotoxicity assays | 954 |
| Neutrophils | |||||
|---|---|---|---|---|---|
| Species | Gradient type | Tissue type | Comments | Downstream application | Ref. # |
| human | discontinuous (1-layer) | whole blood | Neutrophils pelleted in the 1.077 g/ml cushion. | FACS analysis, intracellular Ca++ and superoxide anion measurements | 955 |
| human | discontinuous (2-layer) | whole blood | polymorphonuclear neutrophil (PMN) labeling by immunoflourescence, adherance assay and superoxide assay | 956 | |
| human | discontinuous (4-layer) | peripheral blood | Percoll was used to separate monocytes and lymphocytes. | immunoflourescence and flow cytometry | 957 |
| human | discontinuous (4-layer) | whole blood | Eosinophils and neutrophils were isolated following dextran sedimentation. | flow cytometry and measurement of lactoferrin release | 958 |
| human | discontinuous | blood | Cell preparation was layered onto a Percoll cushion to remove monocytes. After lysis of the erythrocytes, primarly neutrophils, with the remaining cells being predominantly eosinophils. | immunoflourescence studies | 959 |
| human | discontinuous | blood | The neutrophils were > 95% pure. | indirect immunoflourescence, immunoelectron microscopy and FACS analysis, O2 consumption | 960 |
| human | continuous, nonlinear (2-layer) | blood | Percoll was used for subcellular fractionation of azurophil granules, specific granules, gelatinase granules, plasma membranes, and secretory vesicles. | ELISAs for NGAL, gelatinase, lactoferrin and myeloperoxidase | 961 |
| mouse | continuous | peritoneum | An ~97% pure polymorphonuclear neutrophilic leukocyte (PMN) preparation was obtained using Percoll. | electrophoretic analysis, GM-CSF assay, and cell morphology and counts | 70 |
| Eosinophils | |||||
|---|---|---|---|---|---|
| Species | Gradient type | Tissue type | Comments | Downstream application | Ref. # |
| human | discontinuous | peripheral blood | Eosinophils were purified using Percoll gradients followed by immuno-magnetic beads. Using this procedure, the eosinophil purity was always > 95% and the viability was > 98%. | FACS analysis, eosinophil migration assays, Ca++ measurements | 962 |
| human | discontinuous (2-layer) | blood | The recovery of eosinophils was 40 to 60%, the viability > 98% as tested by trypan blue exclusion, and the purity > 85%. | chemotaxis and intracellular Ca++ measurements | 963 |
| human | discontinuous (2-layer) | blood | Eosinophil purity was > 95%, and the method did not induce priming of the eosinophils. | serum-treated Zymosan (STZ) binding and placentaactivating factor (PAF) measurments | 964 |
| human | discontinuous (2-layer) | blood | Eosinophil purity was always > 85% and the recovery ranged from 40 to 60%. Viability was > 98%. | chemotaxis assay | 965 |
| human | discontinuous (3-layer) | peripheral blood | Eosinophil purity was 95 to 99%, viability using trypan blue was > 98%, and recovery was 40 to 60%. | density distribution analysis, cell culture | 966 |
| human | discontinuous (4-layer) | whole blood | The effect of dextran sedimentation on the density of neutrophils and eosinophils was analyzed. | flow cytometry and measurement of lactroferrin release | 958 |
| Basophils | |||||
|---|---|---|---|---|---|
| Species | Gradient type | Tissue type | Comments | Downstream application | Ref. # |
| human | continuous | peripheral blood | Basophils were purified by Percoll density gradient separation and cell sorting. The procedure yielded 95% purity with a total yield estimated to range from 5 to 28%. | flow cytometry, histamine release, electron microscopy | 967 |
| human | continuous | bone marrow | The purity of basophils in the low density fraction (< 1.063 g/ml) was generally > 75% of the cells. | histamine content and release | 968 |
| human | discontinuous | peripheral blood | Highly purified basophils were obtained by Percoll gradient followed by negative selection using flow cytometry. | effects of cytokines on human basophil chemotaxis | 969 |
| human | discontinuous (2-layer) | blood | The majority of the basophils were located at the 1.070 to 1.080 interface. The purity in this fraction was 36 to 63%. | further purification by negative selection using immunomagnetic beads | 970 |
| human | discontinuous (2-layer) | blood | Highly purified basophils were obtained by Percoll gradient followed by negative selection using flow cytometry. | histamine release assay, chemotactic assay | 971 |
| human | discontinuous (3-layer) | whole blood | Basophils were purified to > 80% using Percoll gradient followed by treatment with monoclonal antibodies to remove contaminants. | flow cytometry and leukotriene C4 generation following calcium ionophore stimulation | 972 |
| human | discontinuous (3-layer) | peripheral blood | Basophil purity was 85 to 96% using Percoll. | cell stimuli and mediator release assay | 973 |
| rat | discontinuous | blood | further purification by immuno-magnetic beads, immunoflourescence, electron microscopy | 974 | |
To continue reading please sign in or create an account.
Don't Have An Account?