2nd Generation Buchwald Precatalysts
Buchwald precatalysts and ligands are bulky electron-rich dialkylbiaryl phospine-based catalysts and structurally related ligands that improve reactivity in Pd-catalyzed cross - coupling reactions. These highly active reagents have been extensively applied in the synthesis of pharmaceuticals, natural products, polymers, and new materials.
The structure of the dialkylbiaryl ligand plays an important role in the efficiency of the catalysts (Figure 1). We offer a wide range of new and diverse Buchwald ligands for various synthetic applications.
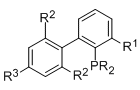
Figure 1.Structural features of dialkylbiarylphosphine ligands
Buchwald precatalysts have the widest scope for Pd-catalyzed C–C, and C–N bond formation. We have introduced a new class of 2nd generation XPhos precatalysts where the parent aliphatic amine is replaced by the more reactive 2-aminobiphenyl (lower pKa) (Figure 2).
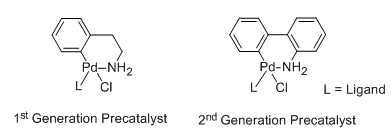
Figure 2.Structure of precatalysts
The Key Features for Precatalysts:
- Air stable
- Highly efficient
- Utilized under mild conditions
- Short reaction times
- Low catalyst loadings
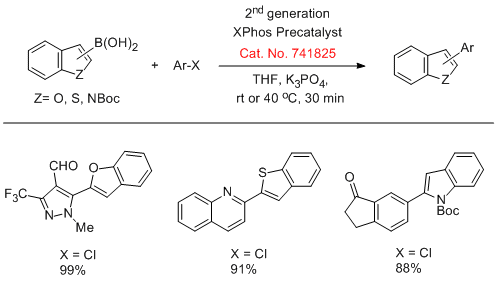
Buchwald and coworkers have reported the use of 2nd generation XPhos precatalysts (Cat. No. 741825) in the Suzuki cross-coupling of unstable polyfluorophenyl and five-membered 2-heterocyclic boronic acids with aryl halides in excellent yields.
Suzuki Coupling of Polyfluoroboronic Acids
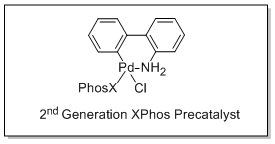
Cat. No. 741825
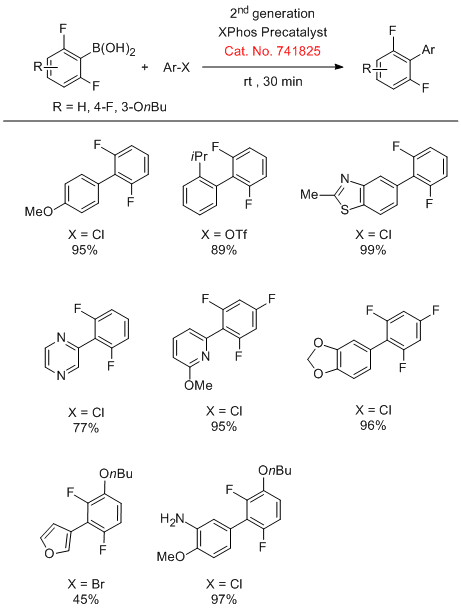
Suzuki Coupling of Heterocyclic Boronic Acids
References
To continue reading please sign in or create an account.
Don't Have An Account?