Hemoglobin & Erythrocytes
We offer a complete line of products and resources related to red blood cell research. Whole red blood cells are available for complement assay and other studies. Purified hemoglobins as well as several heme-related porphyrin compounds are also available.
Section Overview
- Red Blood Cells (Erythrocytes, RBCS)
- Hemoglobin
- Procedure for Reduction of Oxidized Hemoglobin to Form the Oxyhemoglobin Derivative
- Heme Related Compounds
- Related Reagents
- References
We offer a complete line of products and resources related to red blood cell research. Whole red blood cells are available for complement assay and other studies. Purified hemoglobins as well as several heme-related porphyrin compounds are also available.

Red Blood Cells (Erythrocytes, RBCs)
RBC Facts:
- Two million new RBCs are formed every second
- Diameter of RBC is 6-8 μM
- Composed of ~90% hemoglobin
- Each RBC contains ~270 million hemoglobin molecules
- RBCs have a life-span of ~120 days before they are removed by the spleen
- Typically 4-8 x 106 cells per ml in normal human blood
We offer glutaraldehyde-fixed sheep RBCs and antibody-sensitized sheep RBCs for complement assays. The antibody-sensitized sheep RBCs have a very short shelf-life and we recommend customers try to plan their assay schedules around our ship dates (approximately every three months).
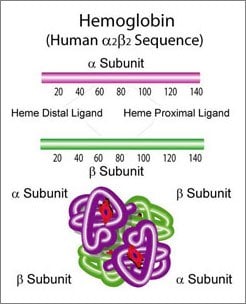
Hemoglobin A, the most common form of hemoglobin, is a tetramer consisting of two 16 kDa α subunits and two structurally similar β subunits, with 141 and 146 amino acid residues, respectively.
Nomenclature and Conversion of Hemoglobin Forms
Oxidized State: Methemoglobin and Ferrihemoglobin are synonymous and refer to the oxidized +3 oxidation state of hemoglobin. Hemoglobin must be in the reduced form in order to bind oxygen.
Reduced State: Ferrous-hemoglobin: can exist as oxyhemoglobin or deoxyhemoglobin both of which are in the reduced +2 form. Oxyhemoglobin contains bound oxygen. Deoxyhemoglobin does not contain bound oxygen.
Procedure for Reduction of Oxidized Hemoglobin to Form the Oxyhemoglobin Derivative
The following protocol is recommended and is based on the method of Dixon, H.B.F. and McIntosh, R. Nature, January 28, 213, 399-400, 1967.
- A 25 x 2.5 column of Sephadex G-25 is equilibrated with 0.02 M phosphate buffer, pH 7.0 containing 1 mM EDTA.
- A solution of 0.2 g of sodium hydrosulfite (sodium dithionite) in 2 mL of the same buffer is applied to the column and allowed to drain into the gel with 1 ml of buffer.
- About 10 mL of the hemoglobin sample is applied to the column and eluted with the phosphate buffer.
- The reduced hemoglobin eluent is saturated with oxygen gas and dialyzed against oxygen saturated buffer to eliminate excess dithionite and to completely convert to the oxyhemoglobin derivative.
Heme Related Compounds

Reference
To continue reading please sign in or create an account.
Don't Have An Account?