Improving Antibody-drug Conjugate (ADC) Solubility with Hydrophobic Novel Payloads Using the Chito-oligosaccharide ChetoSensar™
Antibody-Drug conjugates (ADCs) often present solubility challenges due to the hydrophobic properties of the attached payloads; poor solubility can lead to aggregates and impact manufacturability or even pharmacokinetic properties.
In an attempt to overcome this hydrophobicity, ADC developers may resort to reducing the drug-antibody ratio (DAR), a strategy which may result in a loss of efficacy, reduce the therapeutic window, and cause unintended side effects. Other opportunities to mitigate solubility challenges are possible and include changes to the formulation, payload, or conjugation site or addition of a co-solvent.
Unfortunately, these approaches require additional investments, which can increase development risks. If the solubility issue cannot be resolved, the clinical development program may have to be terminated.
Increasing Solubility with Chito-oligosaccharide Technology (ChetoSensar™)
We have developed a chito-oligosaccharide (ChetoSensar™) that dramatically increases the solubility of ADCs when incorporated into the linker-payload construct (Figure 1).
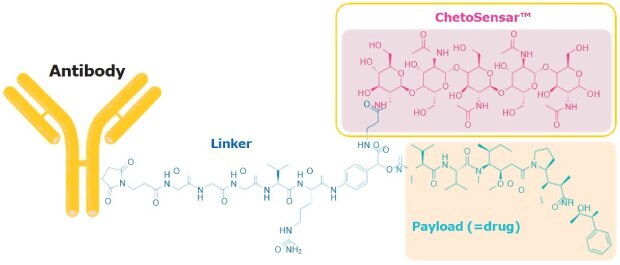
Figure 1.ChetoSensar™ attached to a cathepsin B-sensitive linker and monomethyl auristatin E (MMAE) payload.
Figure 2 shows the reduction in ADC hydrophobicity when ChetoSensar™ is incorporated into the construct. The top panel illustrates the increase in hydrophobicity of a DAR 4 ADC compared to the unconjugated trastuzumab (Herceptin) monoclonal antibody. When the same DAR 4 ADC has ChetoSensar™ attached, a notable shift towards the unconjugated antibody is observed, indicating that the solubility properties of the ChetoSensar™-ADC are now closer to the unconjugated antibody, facilitating ADC processing in development and manufacturing.
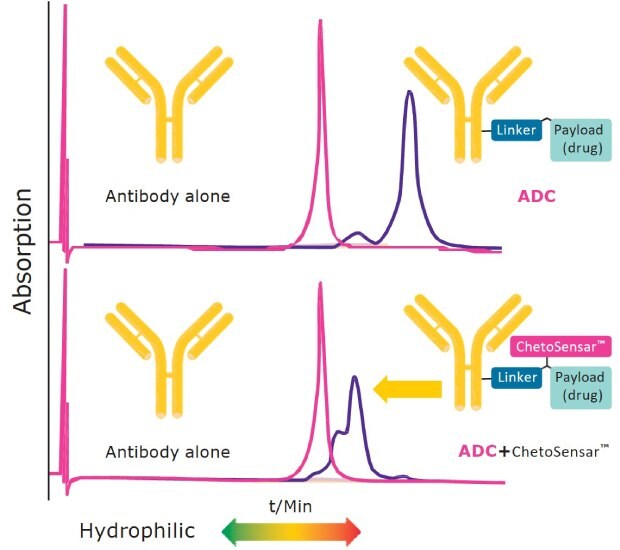
Figure 2.. Incorporating ChetoSensar™ into an ADC reduces hydrophobicity. Shown: hydrophobic interaction chromatogram (HIC) with antibody alone (trastuzumab) and DAR 4 ADC with and without ChetoSensar™ (payload is MMAE).
ChetoSensar™ ADC Achieves Tumor Regression Close to Baseline
To demonstrate the benefit of ChetoSensar™ in an ADC construct, two ADCs were compared in an in vivo study using an SK-OV-3 xenograft (Figure 3). Tumor volume was measured following a single dose of DAR 4 ADC with and without ChetoSensar™. The results showed that at equal doses, the ChetoSensar™ enabled ADC led to rapid and complete tumor regression through day 80, indicating a dramatic increase in efficacy of the ADC.

Figure 3.ChetoSensar™-ADC achieves tumor regression close to baseline in SK-OV-3 xenograph. Both ADCs are DAR 4 at 6 mg/kg. Single dose begins at 31 days. All ADCs use traztuzumab as model mAb. Median results report from a group of eight mice.
In addition to efficacy, tolerability of an ADC is critical to its success as a therapeutic. One indicator of tolerability is the body weight of mice throughout the course of an in vivo study. With DAR 4 (Figure 4A) and DAR 8 (Figure 4B) ChetoSensar™-enabled ADCs, there was no loss in body weight, indicating the ChetoSensar™-ADCs were well tolerated. In addition, no off-target toxicity of ChetoSensar™ was observed. There were no histological changes in any major organs and no immune response created when added to peripheral blood mononuclear cells (PBMCs) that were isolated from three different human donors (data not shown).
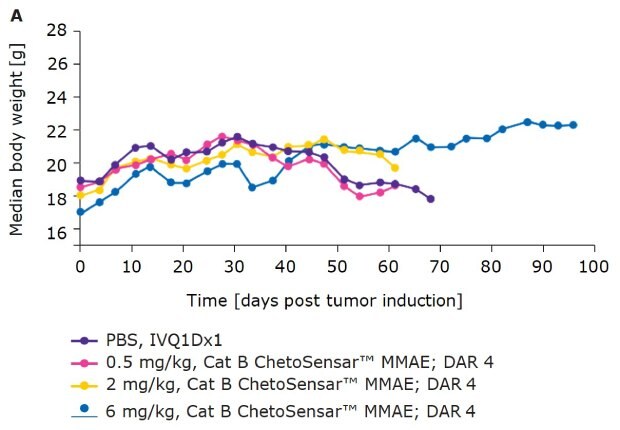
Figure 4a.ChetoSensar™-ADCs were well tolerated in vivo with repetitive. Body weight of mice in animal study of DAR 4 ADCs with ChetoSensar™. No body weight loss or side effects (e.g. skin rash) were observed.
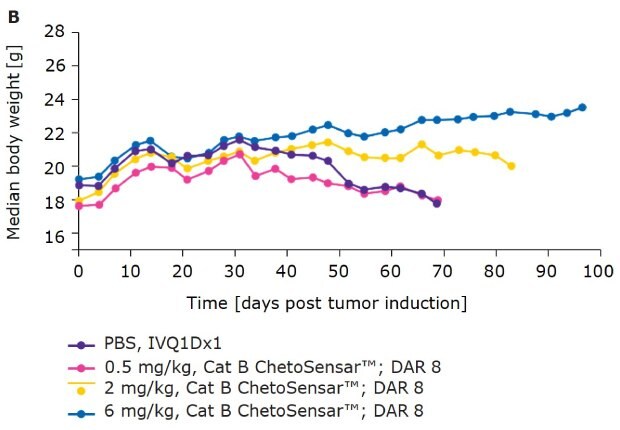
Figure 4b.ChetoSensar™-ADCs were well tolerated in vivo with repetitive. Body weight of mice in animal study of DAR 8 ADCs with ChetoSensar™. No body weight loss or side effects (e.g. skin rash) were observed.
ChetoSensar™ Improved ADC Efficacy
We sought to define the reasons for the increased efficacy of ChetoSensar™-enabled ADCs. These ADCs contain the p-aminobenzyloxycarbonyl (PABC) group that is susceptible to cathepsin B (Cat B) cleavage in the lysosome. PABC linkers have been shown to undergo non-specific clearance and payload loss. However, in vitro studies show that ChetoSensar™ may act as a stabilizer against the mouse enzyme carboxyesterase 1C when attached to PABC.
The monoclonal antibody Herceptin (trastuzumab) in combination with ChetoSensar™ was shown to be stable in mouse and human cells without any nonspecific clearance based on the corresponding carboxylesterase (data not shown). This indicates that in addition to improvements in solubility, the stability of the ADC is driven by improved pharmacokinetics with no negative effect on the binding of the antibody.
ChetoSensar™ Enables Higher Drug-Antibody Ratio ADCs
We then created a site-specific DAR 2 duocarmycin ADC with ChetoSensar™ and tested it in humanized FcRn mice to predict in vivo behavior in humans (Figure 5). The DAR 2 ChetoSensar™-ADC in buffer solution had a potency in the sub-nanomolar range. In vivo plasma samples taken at 4 hours and 168 hours were subsequently tested on antigen-positive cells and showed no loss of efficacy. This indicates that the samples were stable for up to seven days in circulation in humanized FcRn mice.
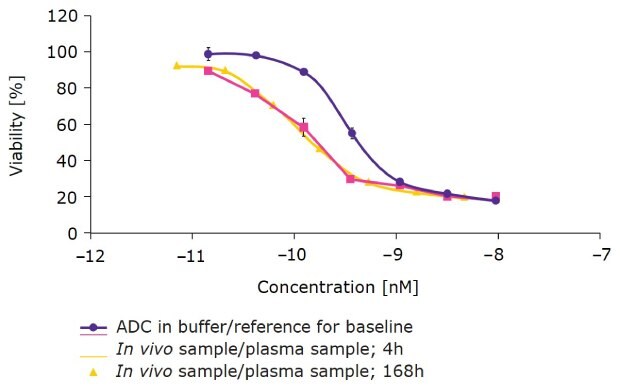
Figure 5.ChetoSensar™-duocarmycin ADC (DAR 2) has favorable pharmacokinetics in humanized FcRn mice. Stability of ChetoSensar™-ADCs is demonstrated with no loss in efficacy after 7 days in antigen-positive cells
Beyond the DAR 2 studies shown here, ChetoSensar™ empowered the preparation of an aggregate-free DAR 8 duocarmycin ADC, which was previously not possible (Figure 6). This reached into higher DAR species with the aid of ChetoSensar™ technology gives optimism to increased potency with other payloads as well.
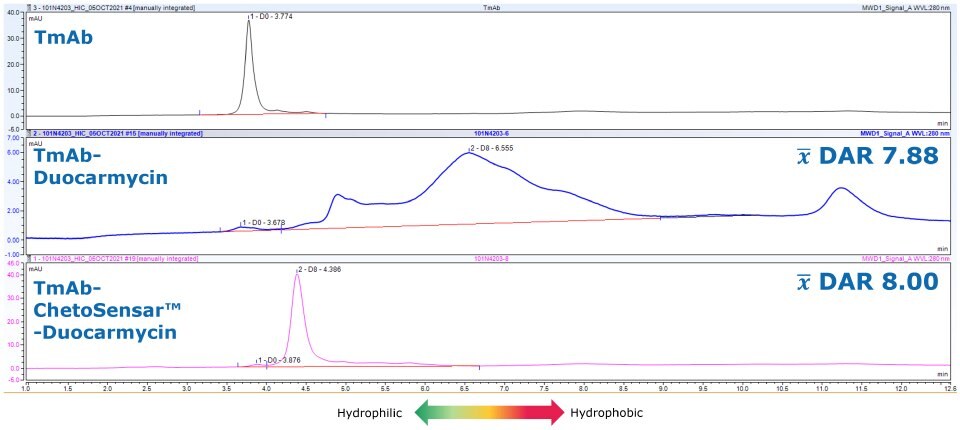
Figure 6.ChetoSensar™-duocarmycin ADC shows no aggregation in HIC compared to sample without ChetoSensar™
This high degree of efficacy, stability, and robust pharmacokinetics also translated to the in vivo setting. As shown in Figure 7, a single dose of DAR 2 ChetoSensar™-duocarmycin ADC led to complete remission of the tumor in a xenograft model with no regrowth through day 100, a notable contrast when compared to the DAR 2 duocarmycin ADC without ChetoSensar™, which had no apparent effect on tumor regression. An ongoing study with a 5 mg/kg dose has also shown complete remission (data not shown).

Figure 7.Demonstrated tumor regression with DAR 2 ChetoSensar™-duocarmycin ADC. Purple: Vehicle (PBS); green: ADC without ChetoSensar™; pink: ADC with ChetoSensar™. Both ADCs are dosed at 10 mg/kg; single dose begins at 0 days. ADC construct design referenced in Figure 5.
Solving the ADC Solubility Challenge
The poor solubility of ADCs can put development programs in jeopardy. ChetoSensar™ technology is an innovative option for addressing these solubility challenges. The chito-oligosaccharide linker offers a great deal of flexibility for attaching different kinds of payloads and has been shown to work with a variety of linkers, antibodies, and conjugation approaches. The technology provides access to highly hydrophobic, novel payloads that might not otherwise be considered and presents the possibility of improved efficacy, tolerability, and pharmacokinetics.
Learn more about our ADC & Bioconjugation CTDMO services.
To continue reading please sign in or create an account.
Don't Have An Account?