900340
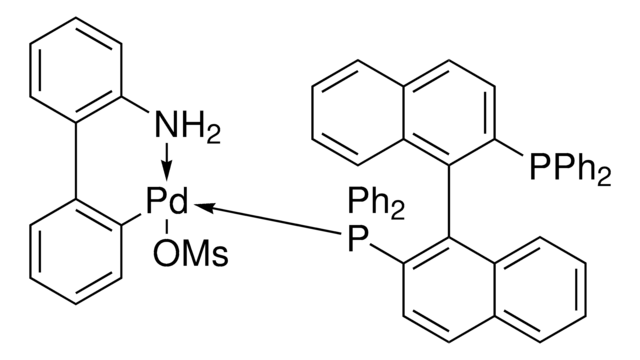
rac-BINAP Pd G4
Synonym(s):
(Methanesulfonatato-κO)[2′-(methylamino-κN)-2-biphenylyl-κC2]palladium - 1,1′-binaphthalene-2,2′-diylbis(diphenylphosphine)
About This Item
Recommended Products
form
powder
Quality Level
feature
generation 4
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
functional group
phosphine
InChI
1S/C44H32P2.C13H12N.CH4O3S.Pd/c1-5-19-35(20-6-1)45(36-21-7-2-8-22-36)41-31-29-33-17-13-15-27-39(33)43(41)44-40-28-16-14-18-34(40)30-32-42(44)46(37-23-9-3-10-24-37)38-25-11-4-12-26-38;1-14-13-10-6-5-9-12(13)11-7-3-2-4-8-11;1-5(2,3)4;/h1-32H;2-7,9-10,14H,1H3;1H3,(H,2,3,4);/q;;;+1/p-1
InChI key
JCAKNOAUMBTXEM-UHFFFAOYSA-M
Application
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
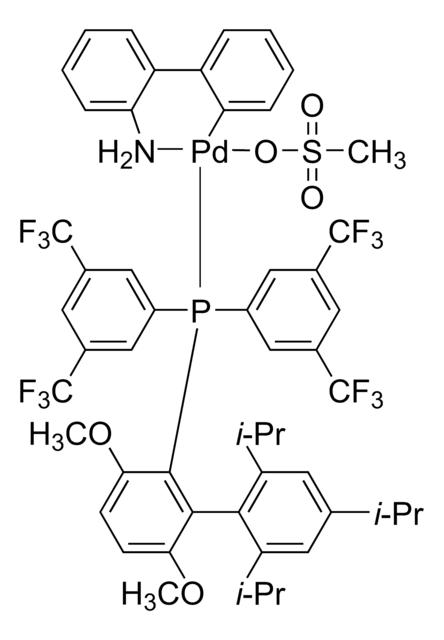
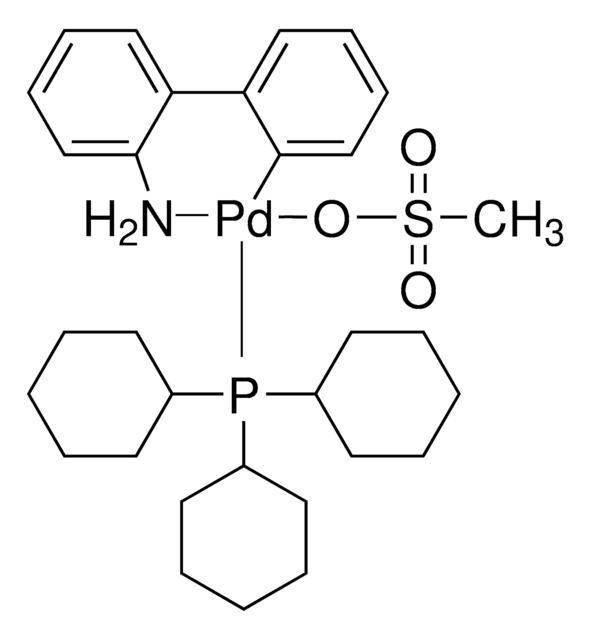
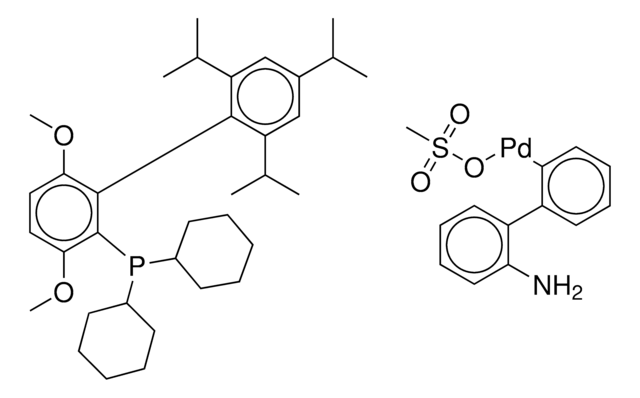
G3 and G4 Buchwald palladium precatalysts are the newest air, moisture, and thermally stable crossing-coupling complexes used in bond formation for their versatility and high reactivity.
Related Content
The Buchwald group has developed a series of highly active and versatile palladium precatalysts and biarylphosphine ligands used in cross-coupling reactions for the formation of C-C, C–N, C–O, C–F, C–CF3, and C–S bonds. The ligands are electron-rich, and highly tunable to provide catalyst systems with a diverse scope, high stability and reactivity. Furthermore, the new series of precatalysts are air-, moisture and thermally-stable and display good solubility in common organic solvents. The use of precatalysts ensures the efficient generation of the active catalytic species and allows one to accurately adjust the ligand:palladium ratio. The ligands, precatalysts and methodology developed in the Buchwald group are user friendly and have rendered previously difficult cross couplings reactions, much easier to achieve.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service