Performing a Separation with HiTrap® IgM Purifcation HP
The technique described here is optimized for purifcation of monoclonal IgM from hybridoma cell culture, but it can be used as a starting point to determine the binding and elution conditions required for other IgM preparations.
Purification Option |
|---|
HiTrap® IgM Purification HP columns are packed with a thiophilic adsorption medium (2-mercaptopyridine coupled to Sepharose High Performance). The interaction between the protein and the ligand has been suggested to result from the combined electron donating- and accepting-action of the ligand in a mixed mode hydrophilic-hydrophobic interaction.
Purification Example
Figure 19 shows results from the purification of monoclonal a-Shigella IgM from hybridoma cell culture supernatant. SDS-PAGE analysis demonstrates a purity level of over 80%. Results from an ELISA (not shown) indicated a high activity of the antibody in the purified fraction.
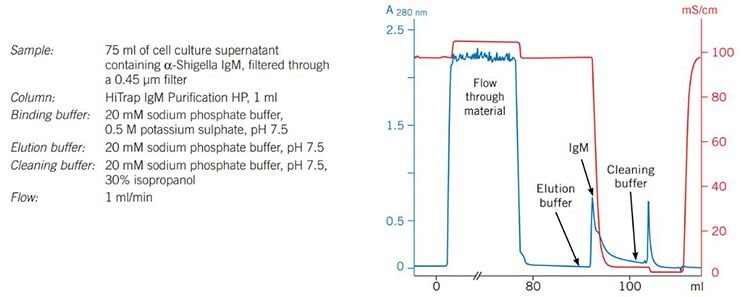
Figure 19a.Purifcation of α-Shigella IgM on HiTrap® IgM Purifcation HP.
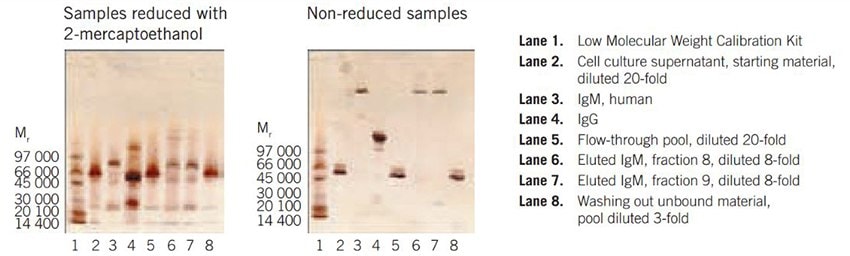
Figure 19b. SDS-PAGE on PhastSystem, using PhastGel 4–15 with silver staining.
Performing a Separation
Column: HiTrap® IgM Purifcation HP
Recommended flow rate: 1 ml/min
Binding buffer: 20 mM sodium phosphate, 0.8 M (NH4)2SO4, pH 7.5
Elution buffer: 20 mM sodium phosphate, pH 7.5
Wash buffer: 20 mM sodium phosphate, pH 7.5 with 30% isopropanol
The sample must have the same concentration of ammonium sulphate as the binding buffer. Slowly add small amounts of solid ammonium sulphate to the sample of hybridoma cell culture supernatant until the final concentration is 0.8 M. Stir slowly and continuously. Pass the sample through a 0.45 µm filter immediately before applying it to the column. Some monoclonal IgM might not bind to the column at 0.8 M ammonium sulphate. Binding can be improved by increasing the ammonium sulphate concentration to 1.0 M.
To avoid precipitation of IgM, it is important to add the ammonium sulphate slowly.
An increased concentration of ammonium sulphate will cause more IgG to bind, which might be a problem if serum has been added to the cell culture medium. If there is IgG contamination of the purified IgM, the IgG can be removed by using HiTrap® Protein A HP, HiTrap® rProtein A FF, or HiTrap® Protein G HP.
Purification
- Wash column sequentially with at least 5 column volumes of binding, elution and wash buffer.
- Equilibrate column with 5 column volumes of binding buffer.
- Apply the sample.
- Wash out unbound sample with 15 column volumes of binding buffer or until no material appears in the eluent (monitored at A280).
- Elute the IgM with 12 column volumes of elution buffer.
- Wash the column with 7 column volumes of wash buffer.
- Immediately re-equilibrate the column with 5 column volumes of binding buffer.
Potassium sulphate (0.5 M) can be used instead of ammonium sulphate. Most monoclonal IgMs bind to the column in the presence of 0.5 M potassium sulphate and the purity of IgM is comparable to the purity achieved with 0.8 M ammonium sulphate.
Some monoclonal IgMs may bind too tightly to the column for complete elution in binding buffer. The remaining IgM will be eluted with wash buffer, but the high content of isopropanol will cause precipitation of IgM. Perform an immediate buffer exchange (Buffer exchange and desalting for affinity chromatography) or dilute the sample to preserve the IgM. Lower concentrations of isopropanol may elute the IgM and decrease the risk of precipitation.
To increase capacity, connect several HiTrap® IgM Purification HP columns in series. HiTrap® columns can be used with a syringe, a peristaltic pump or connected to a liquid chro-matography system, such as ÄKTAprime plus.
Reuse of HiTrap® lgM Purification HP depends on the nature of the sample and should only be considered when processing identical samples to avoid cross-contamination.
Media Characteristics |
|---|
* Long term refers to the pH interval over which the medium is stable over a long period of time without adverse effects on its subsequent chromatographic performance. Short term refers to the pH interval for regeneration, cleaning-in-place and sanitization procedures.
Storage
Wash the column with 5 column volumes 20% ethanol and store at +4 to +8 °C.
To continue reading please sign in or create an account.
Don't Have An Account?