Refolding: Ion Exchange Chromatography
As for IMAC, refolding using ion exchange chromatography involves reversible immobilization of the denatured protein. Immobilization prevents aggregation during the subsequent denaturant removal. While adsorption conditions to an IMAC medium for a histidine-tagged protein are essentially generic, the conditions for adsorbing to an ion exchange medium will depend on the charge characteristics of the target protein. Both cation (e.g., SP Sepharose) and anion exchange (e.g., Q Sepharose) media have been used successfully for refolding. Binding to ion exchange media usually requires low ionic strength conditions (i.e., below 0.1). Strong binding to a cation exchange resin is promoted at a low pH while strong binding to an anion exchange resin is promoted at a high pH.
Examples of refolding using anion exchange and cation exchange chromatography are shown in Figures 3.7 and 3.8, respectively. Experiments can be designed in a number of ways. In Figure 3.7, the refolding buffer was applied to the column and the flow stopped for 0 to 40 h in different experiments. In Figure 3.8, refolding was done using a decreasing urea gradient. In both examples, the refolded protein was eluted using a salt gradient.
Once refolded, the protein may be purified further by other techniques, such as gel filtration, if a higher degree of purity is required and to remove aggregates if necessary.
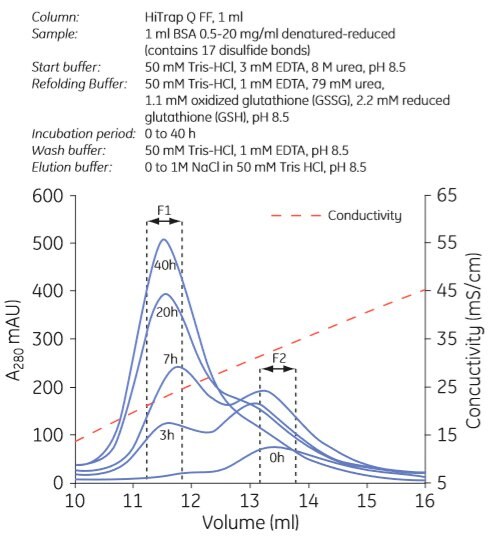
Figure. 3.7. Refolding of albumin using anion exchange chromatography. F1 contains refolded protein. Used with permission. Copyright 2005. Elsevier Science BV.
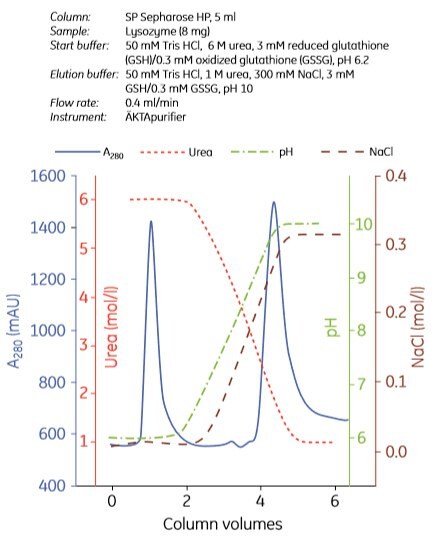
Figure. 3.8. Refolding of lysozyme using cation exchange chromatography. Used with permission. Copyright 2002. Elsevier Science BV.
Suitable prepacked ion exchange chromatography columns for refolding are listed in Table 3.8.
Analysis of refolding
A comprehensive characterization of the outcome of a refolding experiment could include assaying the biological activity and also a physical-chemical characterization of the homogeneity and secondary structure (often using circular dichroism). In many cases, and in particular for proteins with an unknown function, this characterization program is not possible or is impractical to perform.
A homogeneity assay with gel filtration is often sufficient as an indicator of whether refolding was successful. A symmetrical peak corresponding to the expected molecular weight for the protein indicates that refolding has occurred. This assay can be carried out rapidly using the prepacked columns Superdex 75 5/150 GL and Superdex 200 5/150 GL (Table 3.9). These columns can be used for rapid analysis after refolding screening experiments.
To continue reading please sign in or create an account.
Don't Have An Account?