Smart Oligonucleotide Delivery by Multifunctional Block Copolymers
Kotaro Hayashi1, Kanjiro Miyata2
1Kawasaki Institute of Industrial Promotion, 2The University of Tokyo
Material Matters™, 2019, 14.3
- Introduction
- PIC Formation Between Oligonucleotides and Polycations
- Chemical Modifications of PEG-PLys for Reversible Stabilization of PICs
- Chemical Modifications of PEG-PLys for Targeted Oligonucleotide Delivery
- Summary
Introduction
Over the past two decades, polymeric materials have been extensively developed for use as delivery vehicles for small nucleic acids or oligonucleotides such as antisense oligonucleotides (ASO) or small interfering RNA (siRNA).1,2 This application of polymeric materials was made possible by the tendency of polycations to electrostatically associate with anionic oligonucleotides in aqueous media to form a nanocomplex, called polyion complexes (PICs) or polyplexes. These PICs protect oligonucleotides from enzymatic degradation and promote their cellular uptake (or adsorptive endocytosis), because the positively charged PIC domain binds to the negatively charged cellular membrane. Notably, chemical modifications of the component polycations can dramatically affect the performance of PICs, which has motivated polymer chemists and engineers to create and test a wide variety of polymers by focusing on biological-environment-responsive chemistry and biocompatibility.3 This work highlights the main features of oligonucleotide-loaded PICs and the current chemical approaches for multifunctionalization of PICs, with the aim of “smart” oligonucleotide delivery.
PIC formation between cligonucleotides and polycations
Poly(l-lysine) (PLys) is one of the most commonly used polycations for preparing oligonucleotide-loaded PICs, both because it is biodegradable and because its pendent primary amines are easily accessible for chemical modifications. PLys with a degree of polymerization (DP) of ~40 can effectively assemble into PICs with oligonucleotides via charge neutralization at approximately charge-equivalent mixing ratios, i.e., an [NH3+ in PLys]-to-[PO3− in oligonucleotide] ratio of approximately 1:1.4 A slight excess of PLys can generate positively charged PICs, which is advantageous for adsorptive endocytosis under typical cell culture conditions. However, positively charged nanoparticles often undergo nonspecific protein adsorption, resulting in the formation of larger aggregates. To avoid aggregate formation, nonionic and hydrophilic poly(ethylene glycol) (PEG) is often conjugated to PLys (or to the PIC surface).5 Typically, diblock copolymers of PEG and PLys (PEG-PLys) are prepared through ring-opening polymerization of Lys(Z) N-carboxyanhydride from amineterminated PEG (PEG-NH2) as a macroinitiator, followed by deprotection of the Z group to afford PEG-PLys.7 The PEG layer sterically stabilize PICs by attenuating both the interaction with charged biomacromolecules such as albumin, and the interaction with neighboring PICs.6
Recently, the PIC formation of PEG-PLys with single-stranded RNA (ssRNA) or double-stranded RNA (dsRNA) was found to follow two-step assembly behavior.8 The primary association was found to be the formation of minimal charge-neutralized units (or unit PICs) comprised of RNA and PEG-PLys. The secondary association was found to be the multimolecular assembly of unit PICs toward micellar (or vesicular) PICs (Figure 1A). The combination of ssRNA (21-mer) and PEG-PLys (molecular weight (MW) of PEG: 2,000–42,000; DP of PLys: ~40) generated unit PICs with a 10 nm hydrodynamic diameter (DH) at concentrations less than 0.01 mg/mL. By contrast, at concentrations greater than the critical association concentration (CAC) of ~0.01 mg/mL, 40 nm PICs, i.e., micellar PICs, were clearly observed, as shown in Figure 1B. This critical increase in size is similar to the conventional association behavior of amphiphilic macromolecules forming polymeric micelles.7 However, the secondary association (or multimolecular assembly) was not observed between dsRNA (21-mer/21-mer) and the same PEGPLys. Unit PICs with a diameter of 10 nm were prepared in a wide range of concentrations between 0.1 and 10 mg/mL (Figure 1B). The different PIC formation modes between ssRNA and dsRNA are explained by the difference in their rigidity: dsRNA has a persistence length of 60 nm and is thus considered a rigid cylindrical molecule, which likely hampers the multimolecular association of unit PICs by decreasing the entropic gain that accompanies the multimolecular association.8

Figure 1. A) Schematic of various PIC formation behaviors between ssRNA (or ASO) and dsRNA (or siRNA) with PEG-PLys. B) Change in the hydrodynamic diameter of PICs prepared from ssRNA or dsRNA with PEG-PLys (MW of PEG: 12,000; DP of PLys: ~40) plotted as a function of concentration.8
On the basis of the PIC formation mode, either type of PICs, i.e., unit PICs or micellar PICs, can be used for delivery of oligonucleotide. We recently demonstrated the strong potential of unit PICs for in vivo systemic oligonucleotide delivery. A long, two-armed PEG-PLys (MW of PEG: 2 × 37 kDa; DP of PLys: ~20) was designed to form 18 nm unit PICs comprised of a single molecule of siRNA or ASO. The PICs exhibited blood circulation stability and efficiently accumulated in murine models of fibrotic pancreatic cancer, presumably because of the excellent tissue permeability associated with their small size as well as the steric repulsive effect derived from the long, two-armed PEG.9 Numerous previous studies have focused on micellar PICs, because their relatively larger size (40 nm ≤ DH ≤ 100 nm) allows them to avoid rapid renal clearance, which has a size threshold of ~10 nm10 when they are systemically administrated. In this regard, one of the most important criteria for polymer design is stability tuning of the micellar states. As previously discussed, multimolecular PICs are likely to dissociate into smaller fragments of unit PICs under diluted conditions (or at concentrations below the CAC). Thus, additional stabilization is necessary to avoid micelle dissociation, but the oligonucleotide payloads ultimately need to be released from the PICs in the target cells. Thus, reversible stabilization of PICs is a prime challenge for systemic oligonucleotide delivery.
Chemical modifications of PEG-PLys for reversible stabilization of PICs
To attain micellar PICs with reversible stability, researchers have integrated biological-environment-responsive linkages into polymeric materials. For example, reduction-environmentresponsive micellar PICs have been fabricated using thiolated PEG-PLys, which includes disulfide crosslinking in the micelle core. Disulfide-crosslinked micelles are relatively stable in nonreductive environments such as the bloodstream, but they are destabilized within the reductive cytoplasmic compartment because the disulfide bonds are cleaved, thereby enabling payload release. The thiolated PEG-PLys is synthesized by introducing thiol groups into PLys side chains using succinimidyl 3-(2-pyridyldithio)propionate (SPDP),11-13 dimethyl-3,3′-dithiobispropionimidate (DTBP),14 or 2-iminothiolane (IT)13,14 (Figure 2A).
The SPDP-derived PEG-PLys (PEG-PLys(MP)) with 10–30% thiol moieties in the PLys side chains was mixed with single-stranded DNA (or ASO) in a buffer solution to form micellar PICs, followed by aerial oxidation to induce disulfide crosslinking in the micelle core. The disulfide-crosslinked micelles exhibited a DH of 40 nm and greater tolerability under nonreductive diluted conditions than noncrosslinked control micelles, and they released the ASO payload under reductive conditions that mimicked the cytoplasm (1 mM glutathione).12
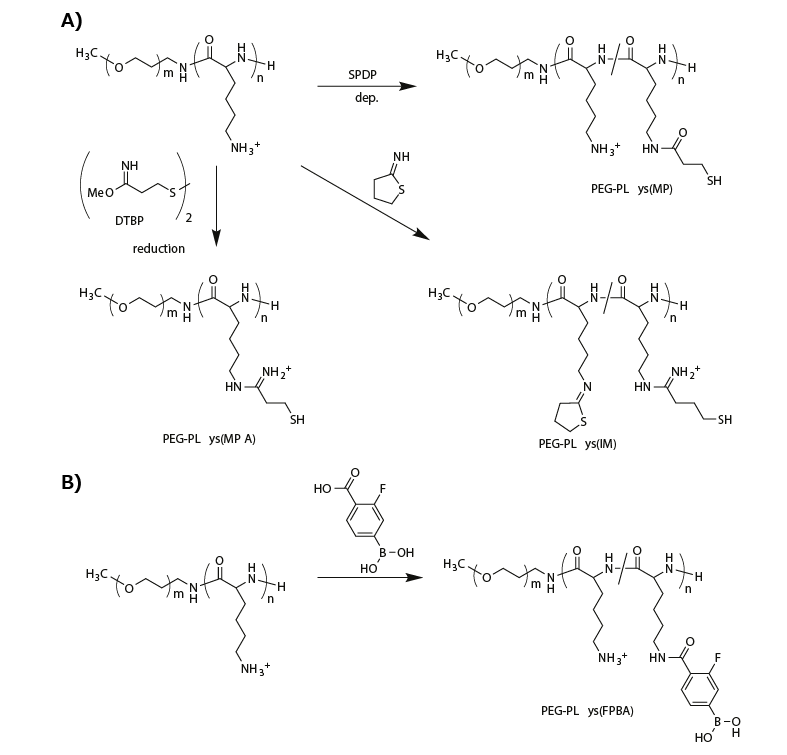
Figure 2.Functionalization of PEG-PLys for fabricating biological-environment-responsive micellar PICs. A) A series of thiolations of PEG-PLys for preparing disulfide-crosslinked micelles. B) Modification of PEG-PLys with phenylboronic acid for ATP-responsive micelle fabrication.
A similar crosslinking strategy was investigated for siRNA-loaded micellar PICs. “Rigid” siRNA-loaded micellar PICs were predicted to be less stable than the ASO-loaded micellar PICs. Thus, more thiol groups (~95% of PLys side chains) or additional stabilizing units were introduced into the PEG-PLys. To this end, DTBP was used instead of SPDP for thiolation of PEG-PLys because it provided a positively charged amidine group and a thiol group (PEG-PLys(MPA)) (Figure 2A). Using this method, the positively charged sites in the PLys segment were maintained even afte such a high degree of thiolation. By contrast, modification with IT provided a closed-ring structure to the PLys segment with amidine and thiol groups (PEG-PLys(IM)) (Figure 2A). This ring structure substantially stabilized the siRNA-loaded micellar PICs, probably because of hydrophobic interactions and/or dipole–dipole interactions. Indeed, the micellar PICs prepared from PEG-PLys(IM) exhibited a substantially longer blood circulation time than those prepared from PEG-PLys(MPA).14
Another interesting approach is the direct, reversible conjugation of siRNA into the PIC core. Tetravalent 3-fluorophenylboronic acid (FPBA) formed a phenylboronate ester bond with a cis-diol moiety at physiological neutral pH.15 Thus, FPBA-functionalized PLys side chains (Figure 2B) can be covalently conjugated with the ribose ring at both 3′ ends of siRNA.16 Importantly, this covalent bond is reversible and can be replaced in the presence of a high concentration of cis-diol compounds, such as, adenosine triphosphate (ATP). When micellar PICs are prepared with PEG-PLys(FPBA) and siRNA, the siRNA payloads are covalently conjugated to the PLys side chains through the phenylboronate ester moiety in the PIC core, generating an siRNA-mediated crosslinked core. Although the FPBA-crosslinked micellar PIC was stable at low ATP concentrations (<0.3 mM, mimicking the extracellular milieu), it successfully dissociated in the presence of high concentrations of ATP (~3 mM, mimicking the cytoplasmic condition). Thus, reversible stabilization can also be achieved using PEG-PLys with phenylboronate functionality.
Chemical modifications of PEG-PLys for targeted oligonucleotide delivery
The PEG layer of a micellar surface can attenuate nonspecific protein adsorption, although it can simultaneously compromise the adsorptive endocytosis (or cellular uptake) of the associated oligonucleotide payloads. To overcome this drawback, functionalized micellar surfaces with ligand molecules for targeted oligonucleotide delivery have been developed.17 Various ligand molecules, including small molecules, peptides, and even antibodies, have been coupled to the terminus of PEG to selectively bind to specific proteins or sugars that are overexpressed on the target cellular surface. Notably, the ligand-installed micellar surface enabled multivalent binding between the micellar PIC and the target cellular surface, dramatically amplifying the binding affinity compared to singlemolecular binding.18
A cyclic RGD peptide (cRGD) was introduced onto the micelle surface (or α-end of the PEG segment in PEG-PLys) to actively target αVβ3 and αVβ5 integrins, which are overexpressed on the surface of various cancer cells and cancer-associated endothelial cells. The cRGD peptide was conjugated by thiazolidine ring formation between the formyl group in a PEG terminus and a cysteine moiety tethered to a cRGD peptide (Figure 3A).19 The cRGD-conjugated micellar PIC, which was prepared from cRGD-conjugated PEG-PLys(IM), resulted in more efficient cellular uptake of siRNA in cultured cervical cancer cells.20 When systemically administrated into subcutaneous-cervical-cancer-bearing mice, the cRGDconjugated micelle strongly enhanced the tumor accumulation of siRNA payloads as compared to a non-cRGD-conjugated control micelle, resulting in significant antitumor activity.20,21
An antibody fragment for human tissue factor (anti-TF Fab′) was also installed at the PEG terminus of micellar PICs prepared from azide-functionalized PEG-PLys(IM) through copper-free click chemistry (Figure 3A). Notably, TF is overexpressed in various cancer and inflammatory tissues, such as, pancreatic cancer and cancer-surrounding fibroblasts. Thus, anti-TF Fab’ is available for cancer and cancer-associated stromal targeting.22 The anti-TF Fab’-conjugated micelle exhibited stronger binding to the TF-overexpressed pancreatic cancer cells in both monolayer and spheroid cultures. Furthermore, systemic administration of the anti-TF Fab’-conjugated siRNA-loaded micelle significantly suppressed the target gene expression in a subcutaneous model of the pancreatic cancer.23
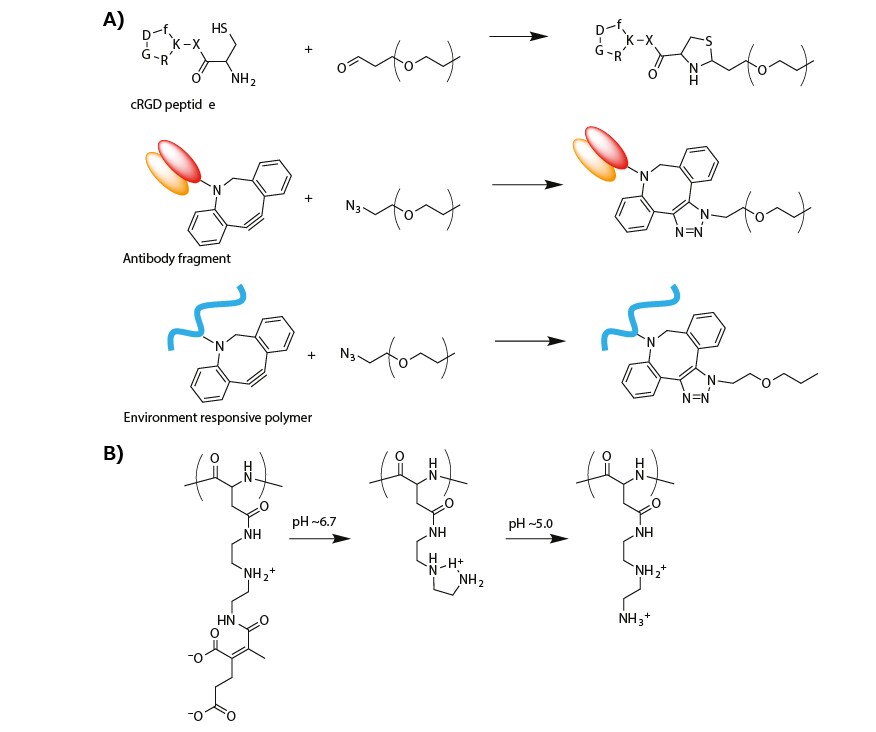
Figure 3. A) Synthesis scheme of modified PEG for targeting delivery of oligonucleotide. B) Change in the chemical structure of PAsp(DET-CDM) induced by biological pH change.
Recently, a targeted PIC micelle conjugated with glucose ligands was reported to transcellularly cross the blood–brain barrier (BBB) and enter the brain parenchyma through recognition by glucose-transporter 1 (GLUT1), which is overexpressed on the brain capillary endothelial cellular surface.24 To this end, a hetero-bifunctionalized PEG with glucose at the α-end and a primary amine at the ω-end (Glu-PEG-NH2) was synthesized by polymerization of ethylene oxide initiated at the 6-position of glucose, and the amination of the other terminus. The systemic administration of the glucose-conjugated micelle led to high PIC accumulation in brain tissue (~6% dose/g brain). This method relies on the GLUT1 recycling mechanism in the brain capillary endothelial cells, which is triggered by a glycemic control method.24 This type of glucose-mediated brain targeting could be used for the systemic delivery of oligonucleotides into diseased brain or glioblastoma.
The biological-environment-responsive functionalization of PEG-PLys was combined with surface modification of micellar PICs to respond to the slightly acidic tumor microenvironment and more acidic endosomal compartment. To this end, a zwitterionic polyasparamide derivative (PAsp(DET-CDM)) was synthesized from diethylenetriamine (DET) and carboxydimethyl maleate (CDM) to alter the chemical (or protonated) structure in a stepwise manner according to a biological pH change.25 Specifically, the CDM moieties were detached from the backbone in response to a slightly acidic tumor microenvironment (pH ≈ 6.7). In addition, the DET moieties moved from the monoprotonated state to the diprotonated state in an acidic late endosomal compartment (pH ≈ 5.0) (Figure 3B). On the basis of this structural change, a PAsp(DET-CDM)-conjugated micellar PIC, which was prepared via copper-free click conjugation, elicited a substantial change in the surface potential from a negative value at pH 7.4, to a modestly positive value at pH ~6.7, for interaction with the negatively charged cellular membrane in tumor tissues. At pH ~5.0, the surface potential exhibited a highly positive value which destablize the endosomal membrane in the cancer cells. Compared with a nonconjugated control micelle, the PAsp(DET-CDM)-conjugated micelle induced efficient gene silencing in cultured lung cancer cells, especially under cell culture condition of pH 6.7.25
Summary
This review described oligonucleotide-loaded PICs prepared from PEG-PLys with or without chemical modifications. The micellar PICs exhibit various functionalities according to their specific chemical modifications, and some of the micellar PICs have demonstrated substantial therapeutic effects in murine cancer models by delivering siRNAs and ASOs that target cancerrelated genes. Despite this success in vivo, clinical trials for polymeric oligonucleotide delivery systems remain rare. The current hurdles for oligonucleotide therapeutics in clinical trials are typically rapid renal and liver clearance.26 The stabilization of PIC micelles through chemical modifications could reduce the rate of renal clearance. The liver is considered to preferably uptake or sequester nanoparticles with high positive/negative charges, lipophilicity, or a large particle size (>100 nm).27 We predict that the rational design of polymeric materials for reversible stabilization and/or piloting modification, including both previously reported and developing techniques, will bring micellar delivery systems into clinical trials, and ultimately lead to therapeutic success.
References
To continue reading please sign in or create an account.
Don't Have An Account?