Immunodetection
Winning Westerns Webinar
Perhaps more than any other step in Western blotting, immunodetection conditions are subject to the greatest number of variables, in part because this step depends on antibody:antigen recognition. Our scientists know how tedious it can be to optimize immunodetection parameters; that’s why we developed the vacuum-driven SNAP i.d.® Protein Detection System, cutting the time for blocking, probing, and washing to 30 minutes.
Go ahead, troubleshoot – and to learn more about fast immunodetection, click here.
Click on the immunodetection topics to read about the possible causes and remedies:
- Weak Signal
- No Signal
- Uneven Blot
- Speckled Background
- High Background
- Persistent Background
- High Background (Pertains to rapid immunodetection protocol only)
- Nonspecific Binding
- Reverse Images on Film (White Bands on Dark Background)
- Poor Detection of Small Proteins
- Presence of Additional Band(s) or Band(s) at the Wrong Size
- Large Error Bars in Quantitative Western Blotting
Weak Signal | |
|---|---|
| Possible Cause | Remedy |
Improper blocking reagent |
|
Insufficient antibody reaction time |
|
Insufficient signal amplification |
|
Antibody concentration is too low or antibody is inactive |
|
Antibody not suitable for Western blotting or not compatible with preparation of cells/tissue |
|
Outdated detection reagents |
|
Protein transfer problems |
|
Dried blot in chromogenic detection |
|
Tap water inactivates chromogenic detection reagents |
|
Azide inhibits HRP |
|
Antigen concentration is too low |
|
No Signal | |
|---|---|
| Possible Cause | Remedy |
| Incompatible primary and/or secondary antibodies | Whenever possible, ensure that the primary antibody is raised against the sequence of the protein found in the same species as your sample. For example, use an antibody targeting the human form of your protein if measuring protein levels in human tissue. If an identical sequence is not possible, determine sequence overlap and reactivity at http://www.ncbi.nlm.nih.gov/protein. Secondary antibodies target the animal species that the primary antibody was raised in. Only use a secondary antibody that was raised in a different species than the host species of the primary antibody, and that is as phylogenetically far from the species of your sample as possible. In addition, ensure that the primary and secondary antibody classes are matched. Most primary antibodies belong to the IgG class, and should be used with a secondary antibody specific for IgG.
|
Antibody concentration too low |
|
HRP inhibition |
|
Primary antibody was raised against native protein |
|
Detection reagent is not sensitive enough |
|
Uneven Blot | ||
|---|---|---|
| Possible Cause | Remedy | |
 Fingerprints on a Western blot caused by touching membrane without gloves or forceps. | Fingerprints, fold marks or forceps imprints on the blot |
|
Speckled Background | ||
|---|---|---|
| Possible Cause | Remedy | |
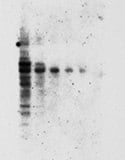 Speckled background Speckled backgroundon Western blot caused by insufficient washes and/or unfiltered blocking solution. | Aggregates in the blocking reagent |
|
Aggregates in HRP-conjugated secondary antibody |
| |
High Background | ||
|---|---|---|
 Overall high background in a Western blot | Possible Cause | Remedy |
Low primary antibody specificity |
| |
Secondary antibody concentration is too high |
| |
Secondary antibody cross-reactivity |
| |
Insufficient washes |
| |
Protein-protein interactions |
| |
Immunodetection on Immobilon®-PSQ transfer membrane |
| |
Poor quality reagents |
| |
Cross-reactivity between blocking reagent and antibody |
| |
Film overexposure |
| |
Membrane drying during incubation process |
| |
Poor quality antibodies |
| |
Excess detection reagents |
| |
Detection reagent is too sensitive |
| |
Persistent Background | |
|---|---|
| Possible Cause | Remedy |
| Nonspecific binding |
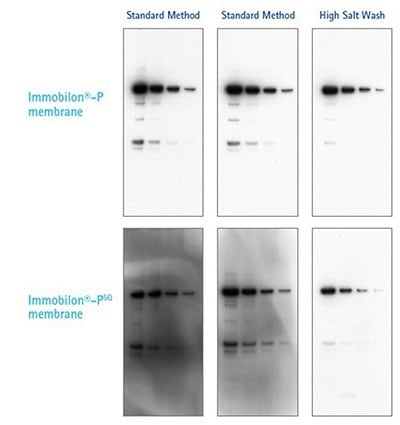 Adding 0.5 M NaCl and 0.2% SDS to the wash buffer reduced the background considerably on the Immobilon®-PSQ membrane, which has a high protein binding capacity. |
High Background (Pertains to rapid immunodetection protocol only ) | |
|---|---|
| Possible Cause | Remedy |
Membrane wets out during rapid immunodetection |
|
Membrane was wet in methanol prior to the immunodetection |
|
Membrane wasn’t completely dry prior to the immunodetection |
|
Nonspecific Binding | |
|---|---|
| Possible Cause | Remedy |
Primary antibody concentration too high |
 Diluting primary antibody increases specificity. |
Secondary antibody concentration too high |
|
Antigen concentration too high |
|
Reverse Images on Film (White Bands on Dark Background) | ||
|---|---|---|
 Negative (reverse) image (“bleached” or “burnt-out” bands) on film caused by excess secondary HRP-conjugated antibody (left). The detection was improved by increasing secondary antibody dilution 10-fold (right). | Possible Cause | Remedy |
Too much HRP-conjugated secondary antibody |
| |
Poor Detection of Small Proteins | |
|---|---|
| Possible Cause | Remedy |
Small proteins are masked by large blocking molecules such as BSA |
|
Presence of Additional Band(s) or Band(s) at the Wrong Size | |
|---|---|
| Possible Cause | Remedy |
Nonspecific primary antibody |
|
Protein degradation |
|
Protein glycosylation, phosphorylation/dephosphorylation, ubiquitination, etc. Protein cleavage |
|
Large Error Bars in Quantitative Western Blotting | |
|---|---|
| Possible Cause | Remedy |
| Variability in blotting conditions between experimental runs |
|
Materials
Loading
Sign In To Continue
To continue reading please sign in or create an account.
Don't Have An Account?