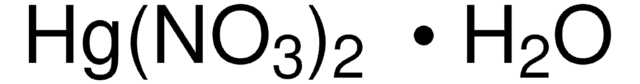TRYPSEQM-RO
Roche
Trypsin Sequencing Grade, modified
from bovine pancreas
Synonym(s):
Trypsin
Sign Into View Organizational & Contract Pricing
Select a Size
All Photos(1)
Select a Size
Change View
About This Item
Recommended Products
biological source
bovine pancreas
Quality Level
form
lyophilized (salt-free)
mol wt
24.000 g/mol
packaging
pkg of 4 × 100 μg (11418033001)
pkg of 4 × 25 μg (11418025001)
manufacturer/tradename
Roche
storage condition
(Keep container tightly closed in a dry and well-ventilated place.)
concentration
0.01-0.2 % (w/w)
technique(s)
protein sequencing: suitable
impurities
Chymotrypsin
General description
Trypsin Sequencing Grade, modified, is isolated from bovine pancreas as a highly purified and specific protease, and subsequently modified.
Trypsin is a highly efficient and specific protease widely used in proteomics for protein digestion. It produces short peptides with specific characteristics that are compatible with current separation and identification methods such as liquid chromatography, mass spectrometry (MS).
Inhibitors: TLCK, DFP, PMSF, leupeptin, soybean trypsin inhibitor, trypsin inhibitor from hen egg, aprotinin, α2-macroglobulin,α1-antitrypsin, APMSF, and antipain.
Trypsin is a highly efficient and specific protease widely used in proteomics for protein digestion. It produces short peptides with specific characteristics that are compatible with current separation and identification methods such as liquid chromatography, mass spectrometry (MS).
Inhibitors: TLCK, DFP, PMSF, leupeptin, soybean trypsin inhibitor, trypsin inhibitor from hen egg, aprotinin, α2-macroglobulin,α1-antitrypsin, APMSF, and antipain.
Specificity
Trypsin is a serine endopeptidase. At pH 7.5–9, it specifically hydrolyzes proteins and peptide bonds C-terminally of Iysine and arginine. Amide and ester bonds of Arg and Lys are also cleaved. The specificity of Trypsin Sequencing Grade, modified, is verified with the oxidized B-chain of insulin (insulin Box) as a substrate. High concentrations of Trypsin Sequencing Grade, modified, one part by weight enzyme with 9 parts by weight insulin Box, are incubated for 18 hours to detect traces ofchymotrypsin impurities.
Application
Use Trypsin Sequencing Grade, modified, to generate glycopeptides from purified glycoproteins.
It is used for:
It is used for:
- Protein-structure elucidation
- Tryptic mapping
- Fingerprinting analysis
- Sequence analysis
- Translocation studies
- Protein identification
- Protein digestion during lipoprotein preparation for liquid chromatography-tandem mass spectrometry (LC-MS/MS)
Quality
Purity: Free of impurities that may interfere with the separation of peptides in reversed-phase HPLC.
Preparation Note
Working concentration: 1/100 to 1/5 of the protein by weight
Storage conditions (working solution): -15 to -25 °C
Trypsin Sequencing Grade, modified, is more resistant to autolysis, even at pH values in the neutral and weakly basic range. The enzyme can be used in high concentrations.
A solution in 1% acetic acid or 1 mM HCI can be used for up to one week when stored at 2 to 8° C. Stored in aliquots at -15 to -25 °C, the solution is stable for at least one year without loss of activity.
Storage conditions (working solution): -15 to -25 °C
Trypsin Sequencing Grade, modified, is more resistant to autolysis, even at pH values in the neutral and weakly basic range. The enzyme can be used in high concentrations.
A solution in 1% acetic acid or 1 mM HCI can be used for up to one week when stored at 2 to 8° C. Stored in aliquots at -15 to -25 °C, the solution is stable for at least one year without loss of activity.
Storage and Stability
Store dry
Other Notes
For life science research only. Not for use in diagnostic procedures.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Xuchu Wang et al.
Molecular & cellular proteomics : MCP, 12(8), 2174-2195 (2013-05-11)
Thellungiella halophila, a close relative of Arabidopsis, is a model halophyte used to study plant salt tolerance. The proteomic/physiological/transcriptomic analyses of Thellungiella plant leaves subjected to different salinity levels, reported herein, indicate an extraordinary ability of Thellungiella to adapt to
Jianke Li et al.
PloS one, 5(10), e13455-e13455 (2010-10-27)
Honeybee (Apis mellifera) exhibits divisions in both morphology and reproduction. The queen is larger in size and fully developed sexually, while the worker bees are smaller in size and nearly infertile. To better understand the specific time and underlying molecular
Comparative proteomics analysis of human FFPE testicular tissues reveals new candidate biomarkers for distinction among azoospermia types and subtypes.
Davalieva, et al.
Journal of proteomics, 267, 104686-104686 (2022)
Scott J Walmsley et al.
Journal of proteome research, 12(12), 5666-5680 (2013-10-15)
Trypsin is an endoprotease commonly used for sample preparation in proteomics experiments. Importantly, protein digestion is dependent on multiple factors, including the trypsin origin and digestion conditions. In-depth characterization of trypsin activity could lead to improved reliability of peptide detection
Proteomics Using Protease Alternatives to Trypsin Benefits from Sequential Digestion with Trypsin
Dau T, et al.
Analytical Chemistry, 92(14), 9523?9527-9523?9527 (2020)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service