おすすめの製品
品質水準
アッセイ
99%
フォーム
powder
mp
189-192 °C (lit.)
SMILES記法
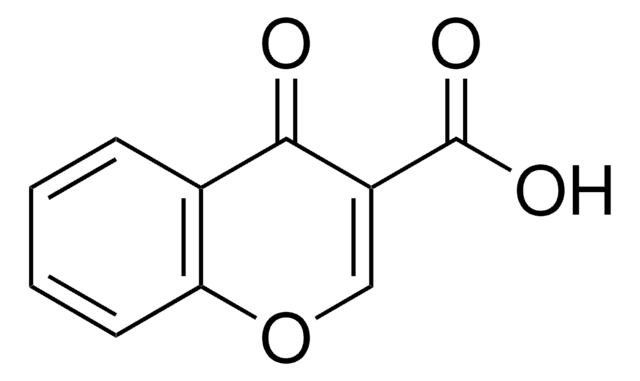
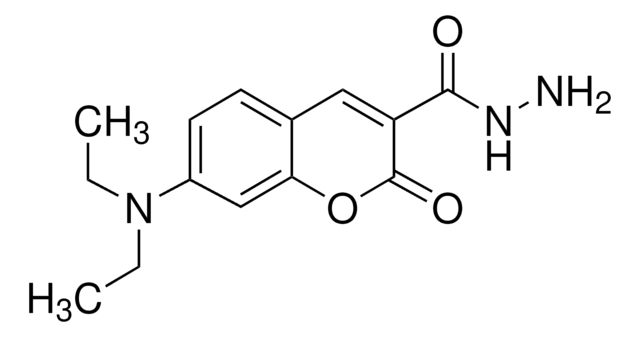
OC(=O)C1=Cc2ccccc2OC1=O
InChI
1S/C10H6O4/c11-9(12)7-5-6-3-1-2-4-8(6)14-10(7)13/h1-5H,(H,11,12)
InChI Key
ACMLKANOGIVEPB-UHFFFAOYSA-N
遺伝子情報
human ... PTPN1(5770)
類似した製品をお探しですか? 訪問 製品比較ガイド
シグナルワード
Danger
危険有害性情報
注意書き
危険有害性の分類
Acute Tox. 3 Oral
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
C85603-5G:
C85603-VAR:
C85603-25G:
C85603-BULK:
C85603-1KG:
C85603-100G:
この製品を見ている人はこちらもチェック
A Karaliota et al.
Journal of inorganic biochemistry, 84(1-2), 33-37 (2001-05-02)
The copper(II) complex of coumarin-3-carboxylic acid (CcaH) has been prepared and characterized on the basis of elemental and thermal analysis, IR, Raman, EPR, UV-Vis reflectance and 1H-NMR spectra. A detail analysis of all spectra data is presented with particular emphasis
Amarjit Singh et al.
International journal of radiation biology, 84(12), 1001-1010 (2008-12-09)
To synthesize N-(3-(3-aminopropylamino)propyl)-2-oxo-2H-chromene-3-carboxamide (7), a novel DNA-binding, coumarin-based, fluorescent hydroxylradical ((*)OH) indicator and to assess its quantum efficiency compared with that of coumarin-3-carboxylic acid (1) and N1,N12-bis[2-oxo-2H-chromene-3-carbonyl]- 1,12-diamine-4,9-diazadodecane (9). Using computer-generated molecular modeling, 7 and 9 and their respective 7-hydroxylated
Rajsekhar Roy et al.
ACS omega, 5(30), 18628-18641 (2020-08-11)
In Alzheimer's disease (AD), insoluble Aβ42 peptide fragments self-aggregate and form oligomers and fibrils in the brain, causing neurotoxicity. Further, the presence of redox-active metal ions such as Cu2+ enhances the aggregation process through chelation with these Aβ42 aggregates as
M A Ali et al.
Free radical research, 32(5), 429-438 (2000-04-15)
The effect of lactic acid (lactate) on Fenton based hydroxyl radical (*OH) production was studied by spin trapping, ESR, and fluorescence methods using DMPO and coumarin-3-carboxylic acid (3-CCA) as the *OH traps respectively. The *OH adduct formation was inhibited by
Grigory V Andrievsky et al.
Free radical biology & medicine, 47(6), 786-793 (2009-06-23)
Aqueous solutions of highly stable supramolecular donor-acceptor complexes of chemically nonmodified pristine C(60) fullerene molecules with H(2)O molecules (hydrated C(60) fullerene-C(60)HyFn) and their labile nano-sized clusters were examined for their antioxidant effects on removal of hydroxyl radicals (.OH) and protecting
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)