おすすめの製品
グレード
certified reference material
品質水準
形状
liquid
特徴
Snap-N-Spike®/Snap-N-Shoot®
包装
ampule of 1 mL
メーカー/製品名
Cerilliant®
drug control
Narcotic Licence Schedule B (Switzerland)
濃度
1.0 mg/mL in methanol
テクニック
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
アプリケーション
clinical testing
フォーマット
single component solution
保管温度
−20°C
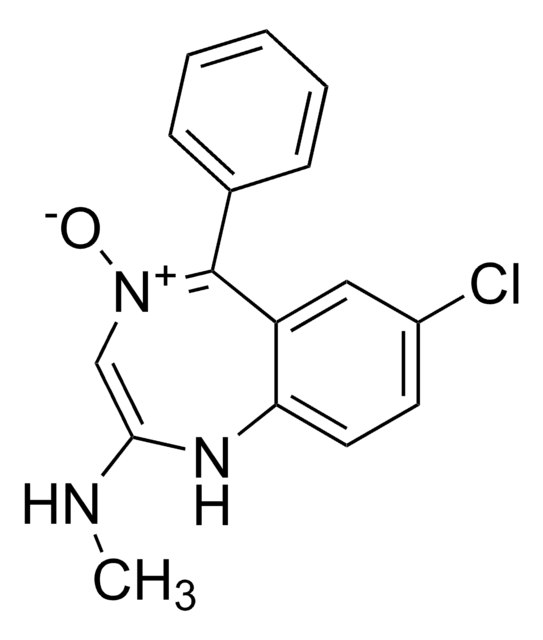
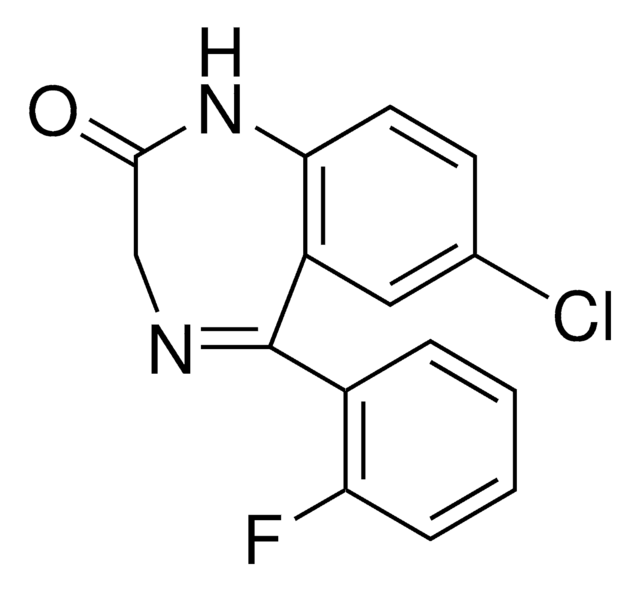
SMILES記法
Fc1ccccc1C2=NCC(=O)Nc3ccc(Cl)cc23
InChI
1S/C15H10ClFN2O/c16-9-5-6-13-11(7-9)15(18-8-14(20)19-13)10-3-1-2-4-12(10)17/h1-7H,8H2,(H,19,20)
InChI Key
UVCOILFBWYKHHB-UHFFFAOYSA-N
詳細
アプリケーション
- Pharmacokinetic profiling: Research on flurazepam metabolites, including Desalkylflurazepam, utilizes high-performance liquid chromatography for detailed pharmacokinetic studies in rats, providing essential data for understanding drug behavior and metabolism (Lau et al., 1987).
- Neuroscience tool: Desalkylflurazepam is applied in neuroscience research to understand the dynamics of benzodiazepine binding on living cells, utilizing small ligands in fluorescence correlation spectroscopy, a method pivotal for real-time molecular interactions study (Hegener et al., 2002).
- Drug testing applications: The compound is used in the development of analytical techniques like ultra-high-performance liquid chromatography-mass spectrometry (UHPLC-MS/MS) for the detection of benzodiazepines in hair, useful in workplace drug testing and forensic science (Ramírez Fernández et al., 2015).
- Toxicological analysis: Desalkylflurazepam serves as a reference standard in forensic toxicology to facilitate the rapid determination of benzodiazepines and their metabolites in biological samples, crucial for accurate and swift diagnostic purposes (Jeong et al., 2015).
- Immunosorbent assay applications: This benzodiazepine metabolite is instrumental in examining cross-reactivity of psychoactive substances in enzyme-linked immunosorbent assay (ELISA) techniques, enhancing drug testing capabilities in clinical settings (Cieri et al., 2024).
法的情報
関連製品
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
ターゲットの組織
Eyes,Central nervous system
保管分類コード
3 - Flammable liquids
WGK
WGK 2
引火点(°F)
49.5 °F - closed cup
引火点(℃)
9.7 °C - closed cup
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
消防法
第4類:引火性液体
アルコール類
危険等級II
労働安全衛生法名称等を表示すべき危険物及び有害物
名称等を表示すべき危険物及び有害物
労働安全衛生法名称等を通知すべき危険物及び有害物
名称等を通知すべき危険物及び有害物
Jan Code
D-915-CC:
D-915-1ML:4548173316499
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)