445874
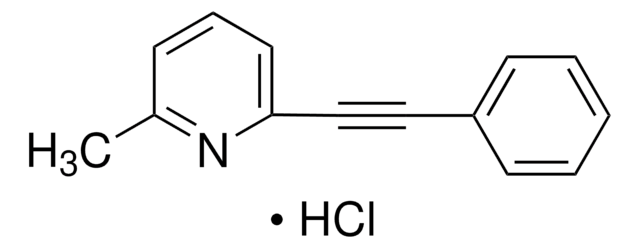
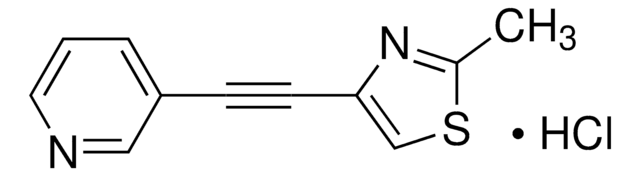
mGluR5 Antagonist, MTEP
The mGluR5 Antagonist, MTEP, also referenced under CAS 329205-68-7, controls the biological activity of mGluR5. This small molecule/inhibitor is primarily used for Neuroscience applications.
別名:
mGluR5 Antagonist, MTEP, 3-((2-Methyl-1,3-thiazol-4-yl)ethynyl)pyridine
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
おすすめの製品
品質水準
アッセイ
≥98% (HPLC)
フォーム
solid
メーカー/製品名
Calbiochem®
保管条件
OK to freeze
protect from light
色
white to yellow
溶解性
methanol: 1 mg/mL
DMSO: 5 mg/mL
輸送温度
ambient
保管温度
2-8°C
SMILES記法
[s]1c(nc(c1)C#Cc2cnccc2)C
InChI
1S/C11H8N2S/c1-9-13-11(8-14-9)5-4-10-3-2-6-12-7-10/h2-3,6-8H,1H3
InChI Key
NRBNGHCYDWUVLC-UHFFFAOYSA-N
詳細
A brain-permeable (thiazole-pyridine)alkyne compound that acts as a potent, selective and non-competitive mGluR5 (metabotropic glutamate receptor subtype-5) antagonist (IC50 = 5 nM in Ca2+-flux assay; Ki = 16 nM) with in vivo anxiolytic activity in a rodent model (ED50 = 1 mg/kg, ip and 7 mg/kg, po). Devoid of any side effects seen with MPEP and benzodiazepines. Reported to weakly affect the activities of other enzymes and receptors tested (IC50 = 30 µM for MAO-A, >100 µM for mGlu1R, 2R & 7R, and >300 µM for NR2BR).
生物化学的/生理学的作用
Cell permeable: yes
Primary Target
mGluR5
mGluR5
Product does not compete with ATP.
Reversible: no
Target IC50: 5 nM in Ca2+-flux assay
Target Ki: 16 nM for mGluR5 (metabotropic glutamate receptor subtype-5)
包装
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
再構成
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
その他情報
Bradbury, M.J., et al. 2005. J. Pharmacol. Exp. Ther.313, 395.
Busse, C.S., et al. 2004. Neuropsychopharmacology29, 1971.
Roppe, J.R., et al. 2004. Bioorg. Med. Chem. Lett.14, 3993.
Klodzinska, A., et al. 2004. Neuropharmacology47, 342.
Cosford, N.D.P., et al. 2003. J. Med. Chem.46, 204.
Brodkin, J., et al. 2002. Eur. J. Neurosci.16, 2241.
Busse, C.S., et al. 2004. Neuropsychopharmacology29, 1971.
Roppe, J.R., et al. 2004. Bioorg. Med. Chem. Lett.14, 3993.
Klodzinska, A., et al. 2004. Neuropharmacology47, 342.
Cosford, N.D.P., et al. 2003. J. Med. Chem.46, 204.
Brodkin, J., et al. 2002. Eur. J. Neurosci.16, 2241.
法的情報
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
保管分類コード
11 - Combustible Solids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
445874-5MG:
445874-200MG:
445874-0MG:
445874-MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Chris S Busse et al.
Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 29(11), 1971-1979 (2004-08-12)
Previous reports have demonstrated the anxiolytic effect of the potent and systemically active metabotropic glutamate subtype 5 (mGlu5) receptor antagonist 2-methyl-6-(phenylethynyl)pyridine (MPEP) in rodents. Here, we present evidence for the anxiolytic activity of a novel mGlu5 receptor antagonist, 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP)
Jeffrey R Roppe et al.
Bioorganic & medicinal chemistry letters, 14(15), 3993-3996 (2004-07-01)
Structure-activity relationship studies leading to the discovery of a new, orally active mGlu5 receptor antagonist are described. The title compound, 5-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-2,3'-bipyridine, is highly potent in vitro, has good in vivo receptor occupancy, and is efficacious in the rat fear-potentiated startle
Metabotropic glutamate receptor mGlu5 is a mediator of appetite and energy balance in rats and mice.
Margaret J Bradbury et al.
The Journal of pharmacology and experimental therapeutics, 313(1), 395-402 (2004-12-14)
The metabotropic glutamate receptor subtype mGlu5 modulates central reward pathways. Many transmitter systems within reward pathways affect feeding. We examined the potential role of mGlu5 in body weight regulation using genetic and pharmacological approaches. Adult mice lacking mGlu5, mGluR5-/-, weighed
Nicholas D P Cosford et al.
Journal of medicinal chemistry, 46(2), 204-206 (2003-01-10)
2-Methyl-6-(phenylethynyl)pyridine (3), a potent noncompetitive mGlu5 receptor antagonist widely used to characterize the pharmacology of mGlu5 receptors, suffers from a number of shortcomings as a therapeutic agent, including off-target activity and poor aqueous solubility. Seeking to improve the properties of
Aleksandra Klodzinska et al.
Neuropharmacology, 47(3), 342-350 (2004-07-28)
Several lines of evidence suggest a crucial involvement of glutamate in the mechanism of action of anxiolytic drugs including the involvement of group I metabotropic glutamate (mGlu) receptors. Given the recent discovery of a selective and brain penetrable mGlu5 receptor
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)